Table of Contents
ToggleTerpenes and Terpenoids are the largest class of secondary metabolites. There arises a question. What do you mean by secondary metabolites? Secondary metabolites are the bioactive compounds which are produced by plants, animals, that are not directly for the life process but used as defensive, attraction for the plants and animals to deal against different conditions. Terpenes are the simplest secondary metabolites which is made up of hydrocarbons. Terpenoids are the modified form of terpenes along with oxygenated groups on different position.
Terpenes and Terpenoids Definition
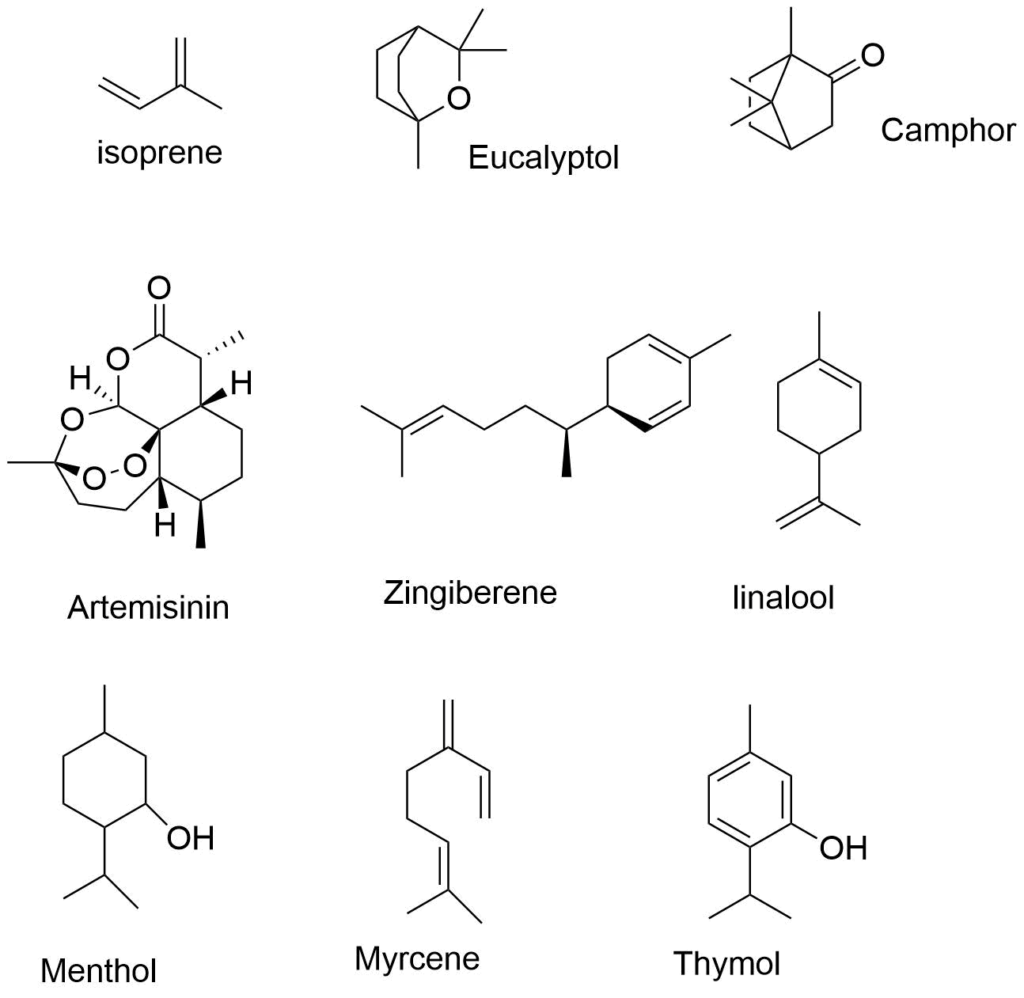
Terpenoids are a large group of natural products in which isoprene is the basic unit. Isoprene is generally denoted by C5H8 and known as 2-methyl-1,3-butadiene. Terpenoids are modified terpenes (isoprenoids) that have had various functional groups added or subtracted and oxidized methyl groups shifted to new places. Terpenes are used in many flavorings and beneficial scents because of their pleasant odor. Terpenoids have a variety of uses in the production of foods, medications, cosmetics, hormones, vitamins, and other products. Terpenoids are found in all parts of higher plants, and occur in mosses, liverworts, algae, lichens, and so on.
Building blocks of terpenoids
The basic unit present in terpenoids is isoprene. It is denoted by C5H8. They are also called as 2-methyl-1,3 butadiene.
Classification of Terpenoids
They are classified on the basis of three factors which are discussed below:
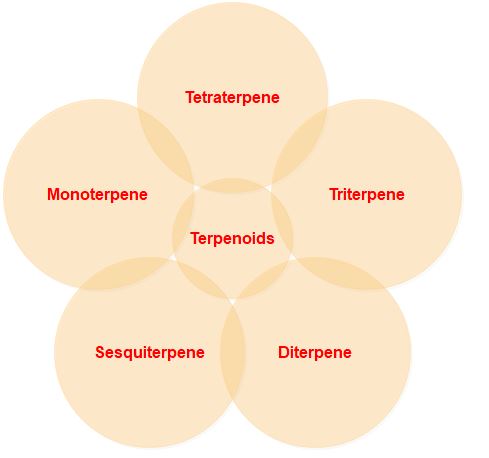
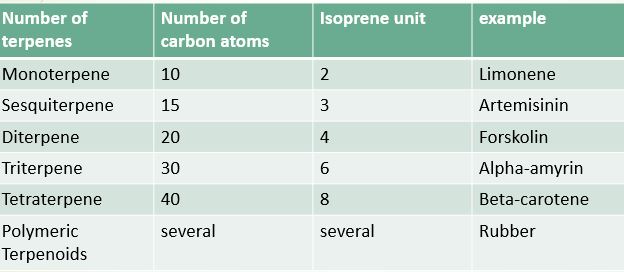
1. On the basis of number of carbon/Isoprene unit
Terpenoids are classified on the basis of the number of carbon/isoprene units, the number of rings, and the functional group.
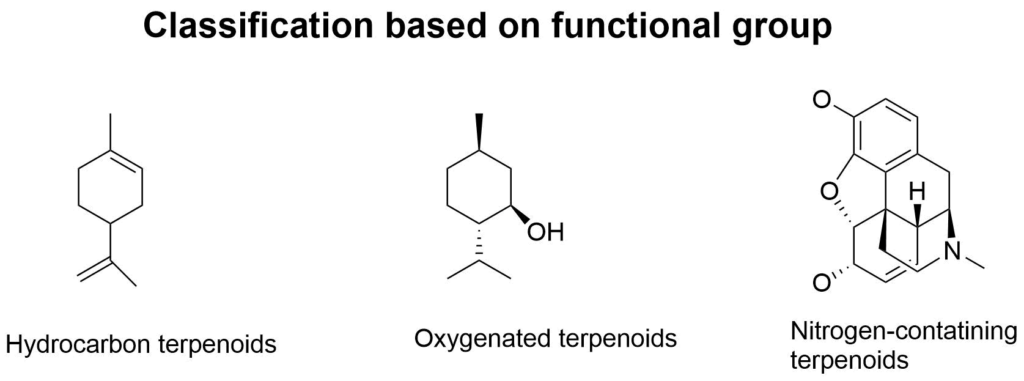
2. On the basis of functional groups
- Hydrocarbons: They contain only carbon and hydrogen
2. Oxygenated terpenoids: They contain oxygen atoms in their structure in the form of ketones, carbonyl groups, alcohols, etc
3. Nitrogen-containing terpenoids: Contain nitrogen in their structure example alkaloids.
3. On the basis number of rings
Ø Acyclic terpenoids: Open structure example: myrcene
Ø Monocyclic: one ring example: limonene
Ø Bicyclic: two rings example: pinane
Ø Tricyclic: three rings
Ø Tetracyclic: four rings
Isolation of terpenoids from volatile oils
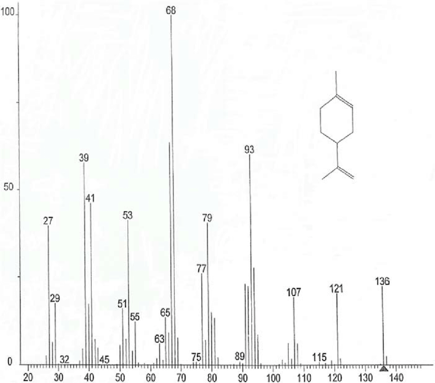
- Terpenoids are generally isolated and identified by using advanced analytical tools called gas chromatography-mass spectrometry.
- On the basis of molecular weight after the separation process from gas chromatography, the structure is identified with the help of mass spectrometry.
- Along with the retention time and m/z ratios, the terpenoids compound can be identified.
- GC produces a chromatogram, and MS produces a mass spectrum in which the graph is plotted against relative abundance Vs. m/z ratio.
- Every time a chemical compound is analyzed, the mass spectrum it produces remains constant, acting as a kind of “fingerprint” that may be used to identify the substance.
- Molecular weight and elemental composition, as well as chemical structure, may be determined using the analytical technique known as MS, which examines the mass-to-charge ratios of charged particles.
- Each peak in a mass spectrum shows a component of unique m/z in the sample and the height of peaks connote the relative abundance of various components in the sample.
- The 3-dimensional mass spectra that are produced by a GC-MS’s data can be utilized to confirm or identify unknown chemicals. For both qualitative and quantitative investigation, the chromatogram can be utilized.
- Overall, GC-MS is a very potent analytical technique since it can identify a molecule not only by comparing its retention time to a standard (GC) but also by evaluating its mass spectrum.
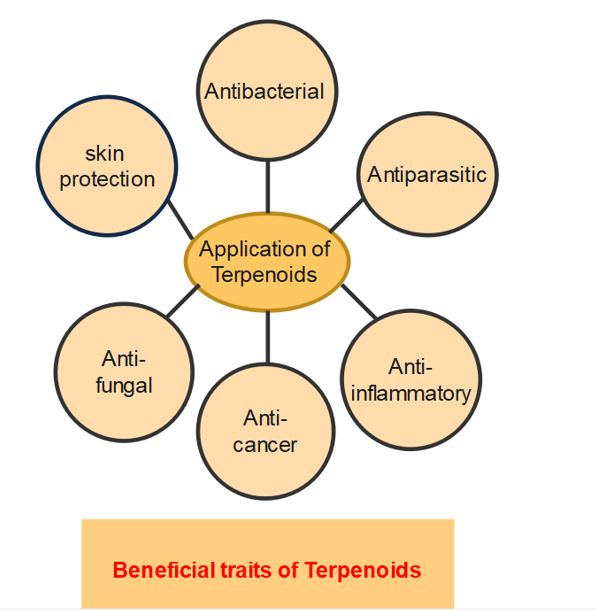
Application of terpenoids
- Terpenes and derived terpenoids, sometimes referred to as isoprenoids, are the most prevalent class of secondary metabolites in plants.
- They play fundamental functions in growth and development as well as more specialized roles in plant-environment interactions, stress resistance, and defense against predators and diseases.
- Can be used as flavoring agents.