Table of Contents
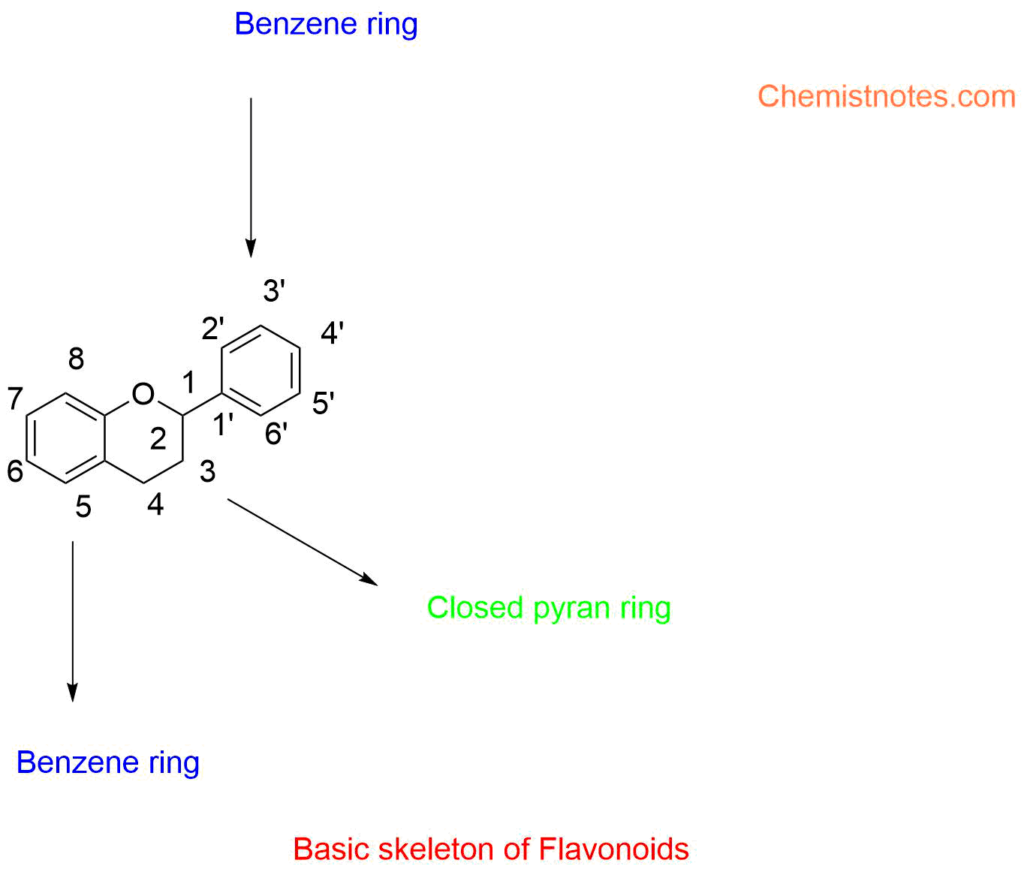
ToggleFlavonoids are a group of polyphenolic secondary metabolites that are present in plants and are thus frequently found in the diets of people. The term “flavonoids” or “bioflavonoids” comes from the Latin word flavus, which refers to their natural color of yellow. Chemically, flavonoids are composed of two phenyl rings (A and B) and a heterocyclic ring (C, the ring with the inserted oxygen), which together make up a 15-carbon skeleton. Plants mostly produce flavonoids, which are secondary metabolites.
Introduction to Flavonoids
Flavonoids have a 15-carbon skeleton in its basic form, with 2 benzene rings joined by a 3-carbon connecting chain. They are therefore shown as C6-C3-C6 compounds. Anthocyanidins, chalcones, flavonols, flavanones, flavan-3-ols, flavanonols, flavones, and isoflavonoids are only a few of the numerous categories of flavonoids that may be categorized based on their chemical makeup, degree of oxidation, and unsaturation of the connecting chain (C3).
History of Flavonoids
Albert Szent-Györgyi and other researchers found in the 1930s that the crude yellow extract from oranges, lemons, or paprika was more efficient in preventing scurvy than Vitamin C alone. The additional ingredients in this combination, which they referred to as “citrin” (relating to citrus) or “Vitamin P” (referring to its impact on lowering capillary permeability), were said to be responsible for the extract’s improved activity. However, it was eventually determined that the chemicals in issue (hesperidin, eriodictyol, hesperidin methyl chalcone, and neohesperidin) did not meet the requirements of a vitamin, rendering this designation obsolete.
Classification of Flavonoids
The seven subclasses of flavonoids include flavonols, flavones, isoflavones, anthocyanidins, flavanones, flavanonols, and chalcones.
1. Flavones
They have a ketone in the fourth position of the C ring and a double bond between positions 2 and 3. According to the taxonomic categorization of the specific vegetable or fruit, certain flavones in vegetables and fruits have hydroxyl groups in position 5 of the A ring, while others have hydroxyl groups in position 7 of the A ring or in positions 3′ and 4′ of the B ring.
2. Flavonols
They differ from flavones in that they have a C ring position 3 hydroxyl group that can also be glycosylated. Like flavones, flavonols exhibit a wide range of methylation and hydroxylation patterns, and given the many glycosylation patterns, they may be the most prevalent and significant subclass of flavonoids in fruits and vegetables.
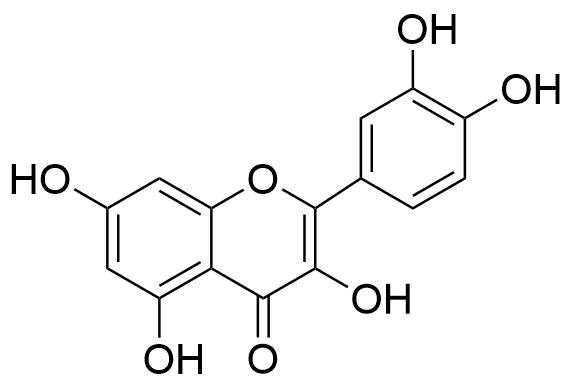
3. Isoflavones
Isoflavones, as one might expect, are a subclass of flavonoids in which the B ring is joined to the third position of the C ring. They are also known as phytoestrogens due to their structural resemblance to estrogens, such as estradiol.
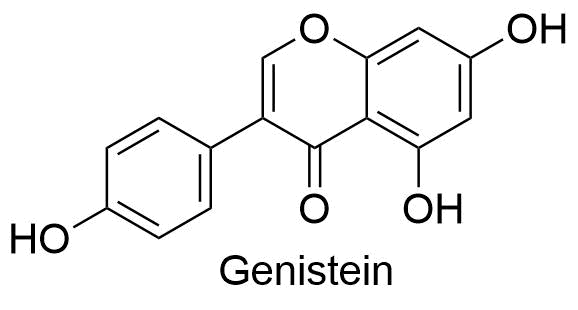
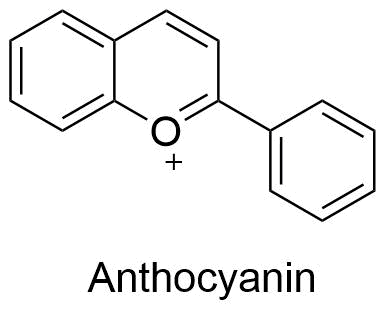
4. anthocyanidins
Anthocyanidins are flavylium cations and are often found as chloride salts in nature.
All other flavonoids are colorless; they are the only class of flavonoids that provide color to plants.
5. flavanones
The main structural difference between the two subgroups of flavonoids is that the double bond between positions 2 and 3 is saturated in flavanones, also known as dihydroflavones, but it is not in flavones.
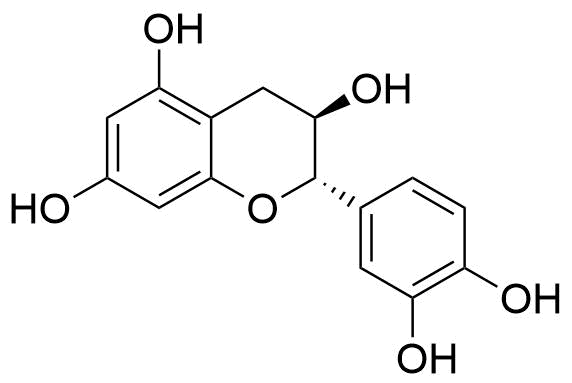
6. flavanonols
The 3-hydroxy derivatives of flavanones are known as flavanonols, and they are a very diverse and multisubstituted class.
7. chalcones
Due to their comparable synthesis routes, chalcones and dihydrochalcones are categorized as flavonoids because of their open structure.
Determination of total flavonoids content from the extracts
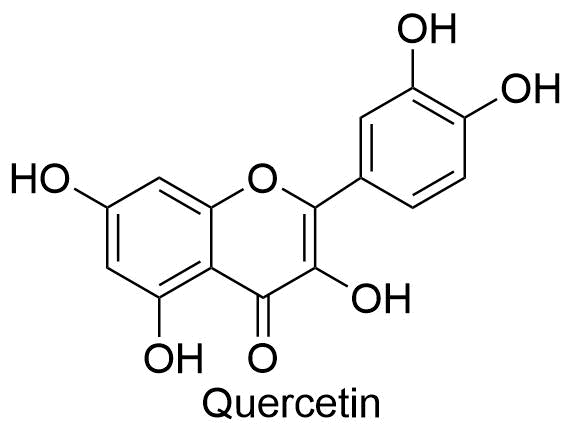
Using the aluminum chloride colorimetric technique, the total flavonoid content is determined. To make a stock solution (4 mg/mL), 1 mL of methanol is used to dissolve 4 milligrams of quercetin. This standard solution is serially diluted to produce 0.25 mg/mL, 0.5 mg/mL, 0.75 mg/mL, and 1 mg/mL solutions at various concentrations. The dose of quercetin is added to the test tube containing 4 mL of distilled water in increments of 1 mL.
This part is already discussed in our previous article, determination of total flavonoids content. You can simply click that and ready that article.
Sources of Flavonoids
The main dietary sources of flavonoids in eastern and western civilizations, respectively, are tea and wine. As additional sources of dietary flavonoids, leafy vegetables, onions, apples, berries, cherries, soybeans, and citrus fruits are regarded as significant sources.
Importance of Flavonoids
Food flavonoids are typically in charge of color, flavor, preventing fat oxidation, and safeguarding vitamins and enzymes. Soy isoflavones, flavonols, and flavones are the flavonoids that are present in the human diet in the greatest concentrations.
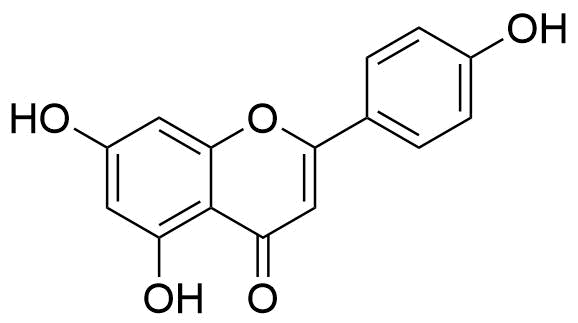
Structure of some flavonoids
The structure of some flavonoids are shown below:
References
http://Clemetson, Alan B. (2018-01-10). Vitamin C: Volume I. CRC Press. ISBN 978-1-351-08601-1.
Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010;2:1231-1246. doi:10.3390/nu2121231