Table of Contents
ToggleJahn Teller put forwarded a theorem that explains why certain six coordinated complexes undergo distortion. Jahn teller effect is observed in octahedral complexes.
Jahn Teller effect
The theorem states that any non-linear molecules or system in an electronically degenerate state will be unstable and will undergo some kind of distortion that will lower its symmetry, remove degeneracy and lower the energy.
The main feature of this system is that it predicts the occurrence of distortion, but does not predict its magnitude.
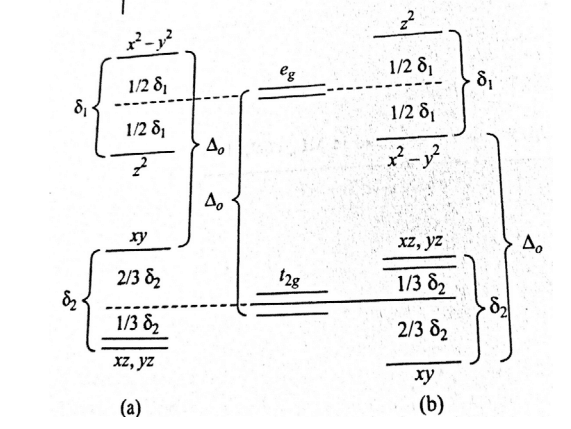
According to this theory, an octahedral complete can not remain at perfect equilibrium but must become distorted in some way. The repulsion of six ligands in the octahedral field split the d-orbital on central metal ion into eg and t2g sets of orbitals. If electrons in d-orbitals are symmetrically distributed between eg and t2g orbital, then they repel all six ligands equally and the geometry of the complex becomes completely regular octahedral.
If the electrons in d-orbitals are asymmetrically arranged between eg and t2g orbital then they repel all six ligands unequally. Thus the structure of the complex is distorted.
Let us take an example of octahedral Cu2+complexes as this effect is most prominent in d9configuration. d9configuration have two possible arrangement of electrons with regard to eg orbitals as shown below
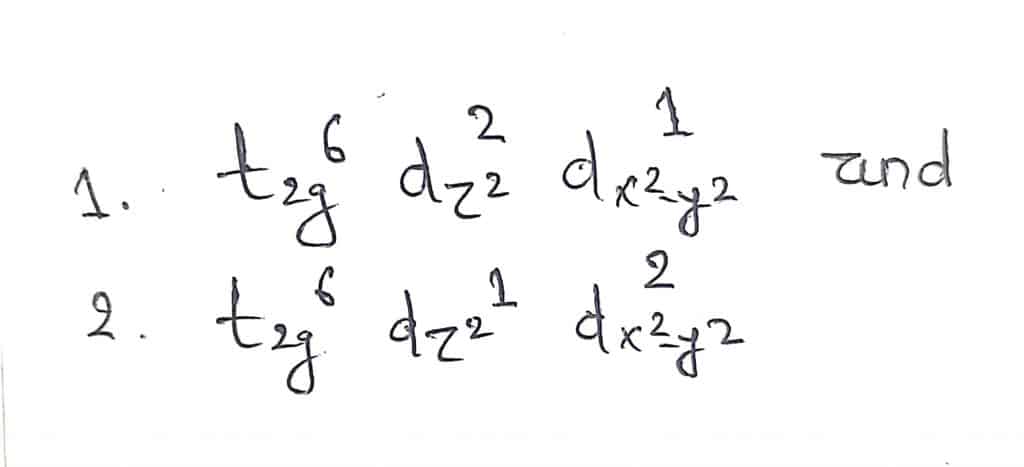
In the first case, ligands along the z-axis are screened more from the electrostatic attraction by Cu2+ than ligands on the x and y-axis. So, the four ligands on the x-y axis are drawn closer than ligands on the z-axis leading to distorted geometry with 2 z-axis ligands elongated.
In the second case, there is exactly an opposite distortion i.e. ligands on the XY plane would move out and those in Z-axis would move in from their equilibrium positions thus we have 2 short bonds along the z-axis and 4 long bonds along the x and y-axis. This type of distortion is called tetrahedral compression which is less common.
The eg level when occupied by an odd number of electrons causes large Jahn-Teller distortions. For many Cu(ii) complexes, the distortion is so large that elongation of the two trans-Z-ligands finally results in square planar complexes.
Jahn Teller effect youtube
Dynamic Jahn Teller effect
In some complexes, distortion can be observed at low temperature but at room temperature, no distortion is observed. Such temperature-dependent phenomenon is called as dynamic Jahn-teller effect.
Dynamic Jahn-teller effect is seen in K2Ph[Cu(No2)6] complex.






