Table of Contents
ToggleCrystal field theory was originally developed to describe the electronic structure of metal ions in the crystal, where they are surrounded by anions that create an electronic field with symmetry dependent on the crystal structure. The crystal field theory was first proposed by Hans Bethe in 1929, which was later modified by J. H. Van Vleck and modified theory is known as Ligand field theory.
Crystal field theory postulates
The basic assumptions of crystal field theory are given below.
- Crystal field theory is an electrostatic approach regrading a cordination complex consisting of central metal ion which is surrounded by a cage of anions or ligands.
- The central metal atom is regarded as cation having charge equal to its oxidation number.
- The ligands are regarded as point charge, which are either negatively charged anions or neutral molecule having lone pairs of electrons.
- The central metal cation is surrounded by a field of anions.
- The bonding in the complexes between metal cation and ligands is purely electrostatic in nature.
- The electrons of metal cation and ligand do not mixup i.e. there is no overlap of orbital between the metal and ligands, which means crystal field theory ignores the covalent bonding between metal and ligand.
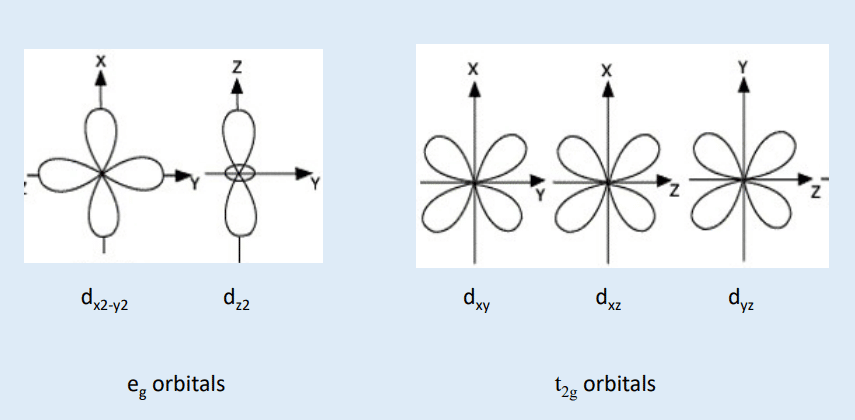
- In an isolated metal ion, all 5d orbitals have same energy and which are termed as degenerate orbital.
- During the approach of ligand towards central metal cation by its negative end, there is repulsion between the electrons of metal ions and that of ligands. Due to this repulsion, splitting of d-orbital takes places place into two sets of d-orbitals eg and t2g having different energies. Such splitting of d-orbital under the influence of electrostatic field of ligand is called as crystal field splitting and its consequences are the heart of Crystal field theory.
- Energy difference between eg and t2g orbital is represented by Δ which is called as Crystal field spliting energy.
Crystal Field Theory Video
Octahedral crystal field theory
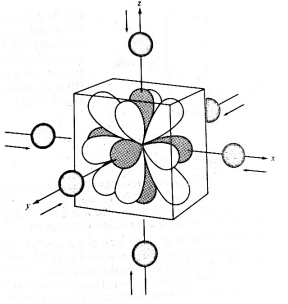
In the case of octahedral complexes, the central metal ion is at the center and the ligands are at the six corners of a cube as shown in the figure. In the octahedral field, the 5d-orbitals split into two sets as
- In the first step, ligand approach the central metal ion from the both ends along the 3 axes producing a hypothetical spherical field which repel all five d-orbitals of metal ions and energy of these orbitals is raised.
- From spherical field, ligands exert octahedral field around the central metal ion, thus octahedral field produce a repulsive force on the electrons of d-orbitals of metal ion. Since eg orbitals are oriented along the axis, the experience more repulsion than t2g orbitals.Therefore, five d orbitals split in to two separate sets of obritals having different energy, which is called crystal field splitting.
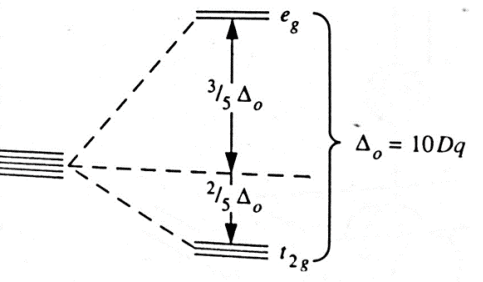
The extent to which the eg and t2g orbitals are separated in an octahedral complex is denoted by Δo (octahedral) or10Dq (crystal field splitting parameter or crystal field strength).
The eg level lie 3/5 Δo above the average level and thet2g level lie 2/5 Δo below the ‘center of gravity,’ or ‘barycenter’ so that the total increase in energy of ‘eg’ electrons is equal to the total decrease in energy of ‘t2g’electron.
Advantages of crystal field theory
Some of the advantages of the crystal field theory are listed below.
- This theory can describe stability of complexes. More crystal field splitting energy, more will be the stability.
- This theory can explain the color and spectra of complexes.
- This theory accounts for the magnetic properties of complexes.
Crystal field theory limitations
The drawbacks of crystal field theory are listed below
- The assumption that the interacton between metal-ligand is purely electrostatic can not be said to be very realistic.
- This theory only takes d-orbitals of central atom in to account. The s and p orbitals are not considered for study.
- This theory fails to explain the behaviour of certain metals which cause large spliting while others show small spliting.
- This theory fails to explain the possibility of having p bonding because P bonding is found in many complexes.
- This theory gives no significance to the orbitals of the ligands. Hence,it can not explain any properties related to ligand orbitals and their interaction with metal orbitals.
Difference between valence bond theory and crystal field theory
The difference between valence bond theory and crystal field theory is given as:
| Valence bond theory | Crystal field theory |
| Valence bond theory describes the formation of covalent bonding. | Crystal field theory describes the electronic structure of coordination complexes. |
| This theory explains the mixing of orbitals. | This theory explains the splitting of orbitals. |
| This theory can not explain absorption spectra. | This can explain the spectra of complexes. |
FAQs:
what is crystal field theory?
Crystal field theory was originally developed to describe the electronic structure of metal ions in the crystal/coordination complexes, where they are surrounded by anions that create an electronic field with symmetry dependent on the crystal structure.






