Table of Contents
ToggleIodine value also called the iodine number is the amount of iodine in grams consumed by 100 grams of a chemical compound. The relative degree of unsaturation in oil components is measured by the iodine value, which is obtained by halogen uptake. Because the degree of unsaturation affects the melting point and oxidative stability, IV offers an estimate of these quality criteria. The more unsaturation and oxidation susceptibility there is, the higher the iodine value. When assessing the level of unsaturation in fats, oils, and waxes, iodine values are frequently utilized.
What is iodine value?
Iodine value is the amount of iodine that 100 grams of a particular substance can absorb. In general words, the amount of iodine that can react with a common mass’s fat is known as the “iodine value” or “iodine number” (100 grams).
Principle of iodine value
Under carefully monitored circumstances, the vegetable oil sample reacts with an excessive amount of iodine monochloride solution (Wijs reagent). In unsaturated fatty acids, primarily oleic and linoleic acids in the case of corn oil halogens quantitatively contribute to the double bonds. Titrating with thiosulfate is used to determine unreacted halogens. The amount of halogen given as iodine in grams that would react with 100 grams of oil is known as the “iodine number.”
Requirements for estimation of iodine value
Chemicals required
Iodine monochloride, Potassium iodide, 0.1N Sodium thiosulphate standardized, 1% starch indicator solution, Chloroform, and Fat sample in chloroform
Materials required
Iodination flask, Reagent bottle, Burette, and burette stand with a magnetic stirrer, Glass pipette, and Measuring cylinder
Procedure of iodine value
- Place the requirements, together with all of the prepared reagent solutions, on the table.
- Pipette 10 ml of a fat sample that has been dissolved in chloroform into a “TEST”-labeled iodination flask.
- Iodine monochloride reagent (20 ml) should be added to the flask. The flask’s contents should be completely combined.
- The flask is then let to stand for a half-hour incubation period in the dark.
- In another iodination flask, create a BLANK by adding 10 ml of chloroform to the flask.
- 20 ml of Iodine Monochloride Reagent should be added to the BLANK, and the contents of the flask should be thoroughly mixed.
- The BLANK should be kept in the dark for 30 minutes.
- After 30 minutes of incubation, remove the TEST from the incubation chamber, and then pour 10 ml of potassium iodide solution into the flask.
- 50 cc of distilled water should be used to rinse the stopper and the flask’s sides.
- Titrate the “TEST” until a pale straw color is seen when it is compared to a standardized sodium thiosulphate solution.
- A purple color is seen after adding around 1 ml of starch indicator to the contents of the flask.
- Till the solution in the flask loses its color and becomes colorless, keep titrating.
- The titration’s conclusion is marked by the blue color’s absence.
- The process is also done for the flask marked “Blank.”
- Note the BLANK’s endpoint values.
Iodine value formula
The iodine value can be calculated using the following formula:
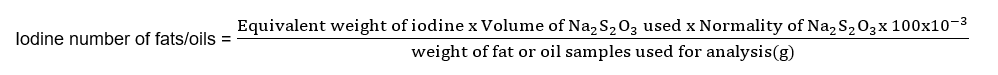
Reaction involved during iodine value test
The reaction involved during the iodine value test is as follows:
ICl + H2O → HIO + HCl
HIO + I– + H+ → H2O + I2
I2 + 2S2O32- → 2I–+ S4O62-
Factors affecting iodine value
Unsaturated oils, fats, and waxes absorb iodine, but saturated oils, fats, and waxes do not, leaving their iodine value zero. (Molecules in unsaturated compounds have double or triple bonds, which are extremely reactive toward iodine).
Does temperature affect iodine value?
Despite the observed variations in the iodine values of the oils, varying heating durations (ranging from 1 to 7 hours) and temperatures (between 27 and 185 °C) had no discernible impact on the iodine levels. Thus, the temperature doesn’t really affect iodine value.
Why iodine value is so important?
Many government rules require that the packaging of food products include a statement regarding the purity of the fats, which is an important factor to measure. The iodine value is the best indicator of the purity of saturated/unsaturated lipids.
Is high iodine value in oil good or bad?
As a result, the locally produced oils’ higher iodine value suggested that they would be better for eating. Due to the danger of cardiovascular disease connected with increased consumption of saturated fatty acids, studies have suggested switching from saturated to unsaturated fats.
Iodine value of some oils
In the paint and varnish industry, drying oils with relatively high iodine values include linseed oil (about 190). Oils that are semi-drying, like soybean oil, contain a range of iodine levels (about 130). Olive oil, a non-drying oil used in culinary goods and soap making, has comparatively low iodine values (about 80).
Saponification value and iodine value
The amount of milligrams of KOH required to neutralize the fatty acids produced by full hydrolysis of 1 gram of an oil sample is known as the saponification value, whereas the Iodine value is the amount of iodine that 100 grams of a particular substance can absorb.
Why is chloroform used in iodine value?
Iodine values are extremely unpredictable and inaccurate when phospholipid is precipitated with acetone and magnesium chloride and chloroform is used as the solvent. This is reportedly caused by the chloroform solution of phospholipid’s propensity to retain significant levels of magnesium chloride.
What is the role of KI in iodine number titration?
Because iodide will oxidize to iodine in the presence of an oxidizing agent, potassium iodide, or KI, is employed in iodometric titration. To modify the color of the solution, the freed iodine is then titrated using sodium thiosulfate.
Application of iodine value
- It is used to determine the unsaturation present in fatty acids, oil, and waxes.
- Unsaturated fatty acid concentration directly correlates with iodine value. It is also used to assess the level of adulteration.
FAQs/MCQs
Which has the lowest iodine value?
Ghee has the lowest iodine values as it is saturated.
Unit of iodine value
The unit of iodine value is grams of iodine absorbed by 100 g of the sample at standard conditions.
Which oil has the highest iodine value?
Soybean oil has the highest iodine value (124–139).
What is the iodine value in Palm oil?
The iodine value of palm oil is 50-55.
Iodine value in activated carbon
The iodine value in activated carbon is 1200 mg/g.