Table of Contents
ToggleEnzyme activity is only visible when it is in continual contact with the substrate. In this post, some major parameters that influence the velocity of the enzyme-catalyzed reactions have been discussed.
Factors affecting enzyme activity
The main factors which affect the rate of the reaction catalyzed by enzymes are listed below.
Concentration of enzyme
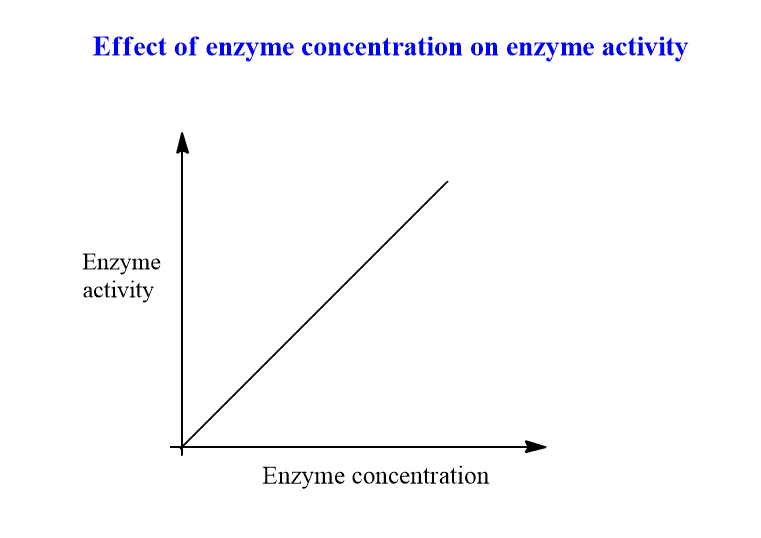
The velocity of the reaction increases according to the concentration of the enzyme. The greater the concentration of enzyme, the more substrate is catalyzed, and hence the rate increases.
Concentration of substrate
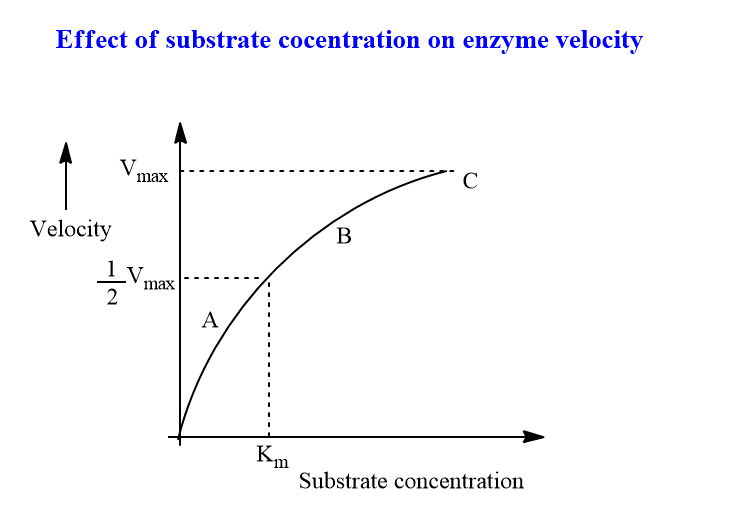
Within the limited range of substrate levels, increasing the concentration of the substrate gradually increases the rate of the enzyme reaction. A rectangular hyperbola is formed when velocity is plotted against the concentration of the substrate.
Temperature
The velocity of an enzyme reaction increases with increasing temperature until it reaches a maximum and then decreases. Typically, a bell-shaped curve is noticed. As the temperature rises, the molecules’ activation energy rises, and there is more molecular enzyme and substrate collision and interaction, allowing the process to proceed more quickly.
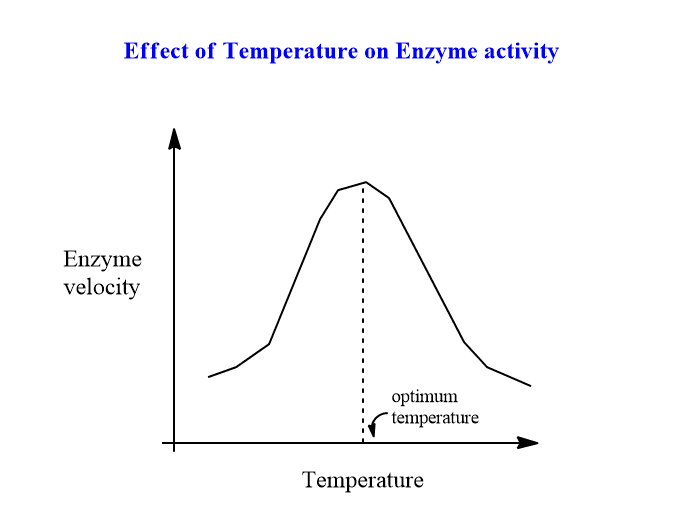
Most enzymes work best at temperatures ranging from 40 to 45 degrees Celsius. However, a few enzymes remain active at temperatures as high as 100 degrees Celsius. This could be because these enzymes have a relatively stable structure and conformation.
When the enzymes are subjected to temperatures above 50 oC, denaturation occurs, resulting in dearangement of the protein’s native tertiary structure and active site.
Effect of pH
When the concentration of hydrogen ions increases, the enzyme activity increases, and a bell-shaped curve is generated. Each enzyme has a maximal velocity at a specific pH. Below this pH, enzyme activity is significantly reduced, and at extreme pH, the enzyme becomes completely inert.
The majority of enzymes in higher organisms perform best at neutral pH. (6-8). This does not rule out the possibility of enzyme function in both acidic and basic pH conditions. Hydrogen ions regulate enzyme activity by changing the ionic charges on amino acids, particularly at active sites, substrates, and ES-complexes.
Effect of product concentration
In general, the accumulation of reaction products reduces the enzyme velocity. For some enzymes, the products interact with the active site and form a loose complex, inhibiting enzyme activity. As a result, this type of inhibition is normally eliminated by removing the product as soon as it is generated.
Effect of activators
Some enzymes require certain inorganic metallic cations for optimal activity, such as Mg+2, Mn+2, Zn+2, Ca+2, Co+2, Cu+2, Na+, K+, and so on. Metals operate as enzyme activators by interacting with the substrate, forming an ES-metal complex, directly participating in the reaction, and causing a conformational change in the enzyme.
There are two categories of enzyme that requires metals for their activity.
a. Metal activated enzyme: The metals are not held tightly by the enzyme and can be exchanged easily with other ions. E.g, ATPase (Mg+2 and Ca+2), Enolase(Mg+2).
b. Metalloenzyme: These enzymes hold the metals tightly and can not be exchanged with other ions. Example: Phenol oxidase( copper), Pyruvate oxidase( Manganese)
Effect of light and radiation
Certain enzymes are turned inactive when exposed to UV, beta, gamma, and x-rays due to the production of peroxidase. Here are several examples: UV rays reduce the action of salivary amylase.