Table of Contents
ToggleZwitterion amino acids vary from each other in isoelectric point or pH. The introduction of zwitterion, isoelectric pH, and amphoteric behavior of amino acids are discussed in this section.
Zwitterion amino acid
The word Zwitter is derived from a German word that means hybrid. Zwitter ion or dipolar ion is a hybrid molecule containing positive and negative ionic groups.
The amino groups generally exist as ionized forms and rarely exist in neutral form with free carboxylic (-COOH) group and free amino groups (-NH2).
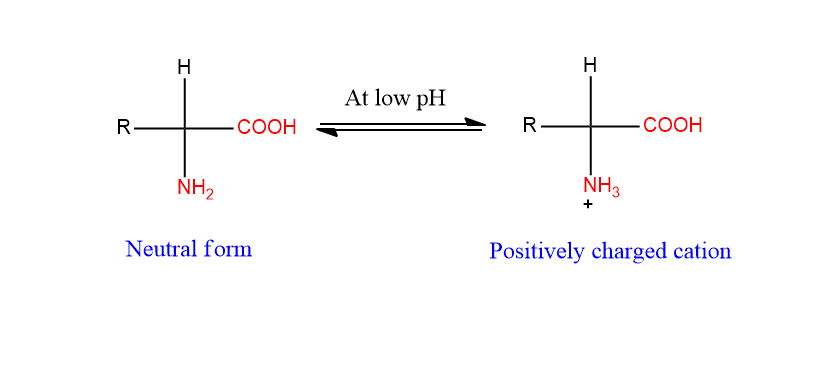
In strongly acidic pH (low pH), the amino group (-NH2) is protonated and hence the amino acid is positively charged cation as shown below.
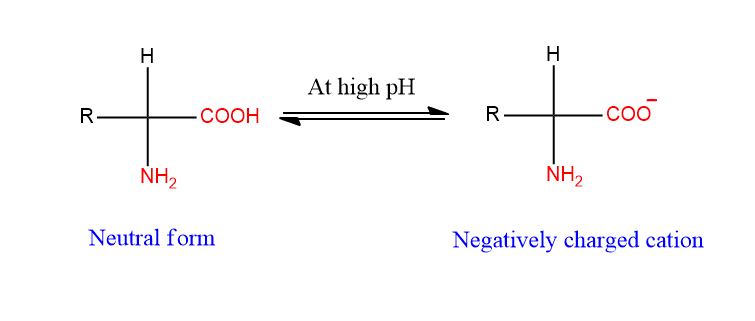
Similarly, In a strongly basic solution (alkaline pH or high pH), the carboxylic group(-COOH) losses a proton (H+), and hence the amino acid is negatively charged anion as shown below.
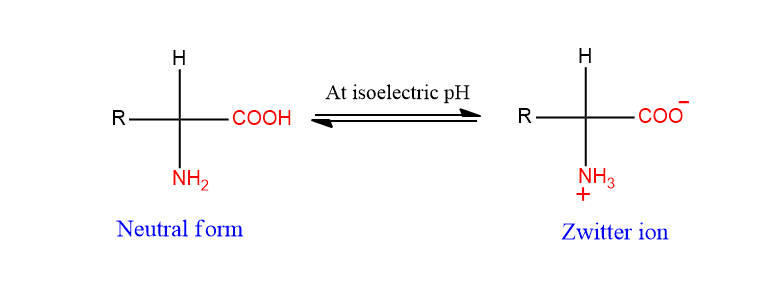
At a certain pH value, an amino acid carries both positive and negative charges and exists as a zwitterion.
Isoelectric point of amino acids
Isoelectric pH (pl) is defined as the pH at which an amino acid exists as zwitterion or dipolar ion and carries no net charge i.e. electrically neutral. For example, Leucine exists as a zwitterion at pH= 6, this pH is termed an isoelectric point for leucine.
The isoelectric pH can be calculated by taking the average pKa values corresponding to ionizable groups. For example, Leucine has two ionizable groups one (-COOH) group and another (-NH2) and its isoelectric point can be calculated as
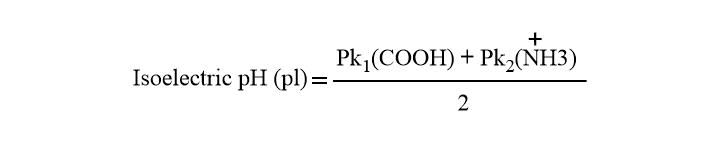
If the amino acids have more than two ionizable groups, we must consider all ionizable groups in order to calculate the isoelectric pH.
Leucine has isoelectric pH =6. What does it mean? It means, that below pH 6, Leucine acts as a positively charged ion, and above pH 6, Leucine exists as a negatively charged ion. At the isoelectric point or pH 6, Leucine exists as a Zwitter ion. Therefore, the pH of the medium determines the ionic nature of the amino acids
Amphoteric nature of amino acids
Amino acids contain both acidic and basic groups. Hence, it can react with both acids and bases. Therefore, amino acids are amphoteric in nature. When an amino acid is treated with a base, it acts as an acid by giving up a proton from the ammonium group.
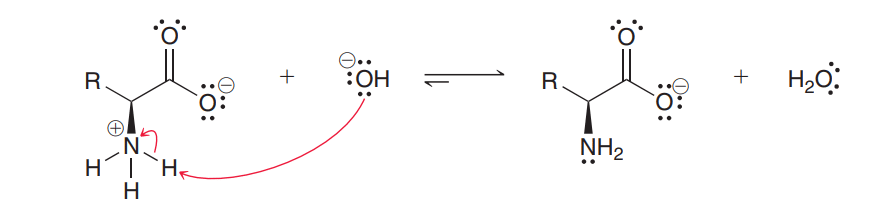
When an amino acid is treated with an acid, it acts as a base by abstracting protons by the carboxylate group.
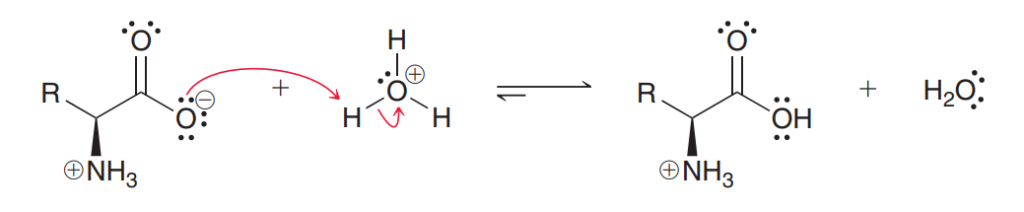
From these reactions, we can conclude an amino acid in its zwitterion form, can function as either an acid or a base.