Table of Contents
ToggleAmines are organic compounds in which alkyl groups are directly attached to nitrogen atoms. It is regarded as the most important organic base structurally related to the inorganic base ammonia. In amines, They are regarded as derivatives of ammonia in which one, two, or all three hydrogen atoms are replaced by an alkyl or aryl group.
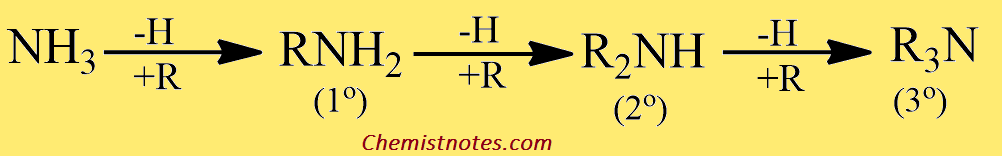
Classification of amines
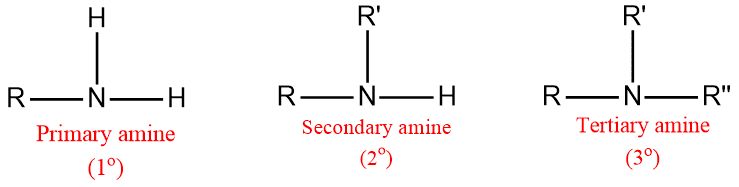
Amines are classified as primary 1º, secondary 2º, and tertiary 3º according to one two, or three organic groups attached to the nitrogen.
- 1o (primary) amine: one carbon atom is attached to the Nitrogen atom of ammonia by the replacement of one hydrogen atom. Example: CH3NH2
- 2o (secondary) amine: Such amine is formed when two organic substituents (carbon atoms) take the place of the two hydrogen atoms in the ammonia molecule. Example: (CH3)2NH
- 3o (tertiary) amine: Three hydrogen atom of the ammonia molecule gets replaced by three carbon atom. Example: N(CH3)3
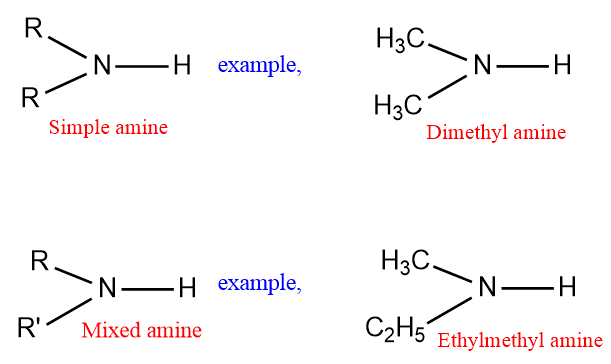
In 2º, and, 3º amines, the groups attached to the nitrogen atoms may be the same or different. Therefore, they may be classified as simple or mixed amines according to the alkyl or aryl groups attached to the nitrogen atom or the same or different.
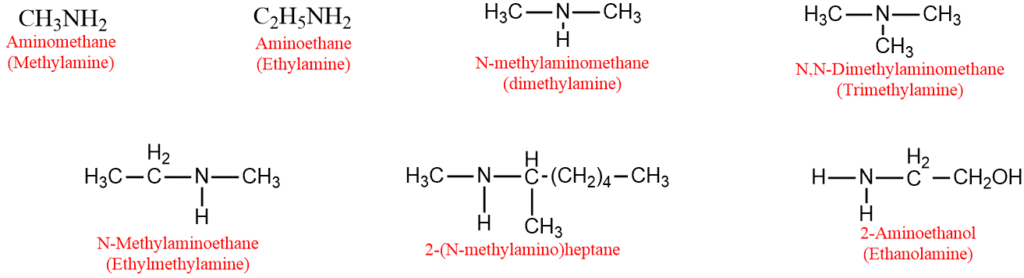
Naming of amines- IUPAC Nomenclature
1. The common name of an aliphatic amine is obtained by adding the suffix amine with the alkyl group. If the alkyl groups attached to the nitrogen atom are identical, the prefixes di or tri are used. If they are different, their names are written in alphabetical order.
2. In the IUPAC system, primary amines are called aminoalkanes. The secondary and tertiary amines are named nitrogen-substituted primary amines are called N-alkylaminoalkanes. The prefix N-alkyl or N, N-dialkyl is written before the word aminoalkane.
3. Amines in which the nitrogen atom is directly attached to an aromatic ring are known as aromatic amines. Aniline is the simplest aromatic amine. Aromatic amines are generally named as the derivative of aniline. An aminotoulene has the special name-toluidine.
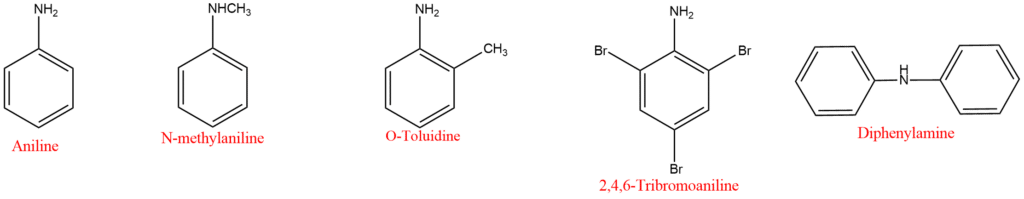
4. Salts of amines are generally named by substituting “amine” with “ammonium” or “aniline” with “anilinium” and adding the name of the anion such as chloride, sulphate etc. For example,
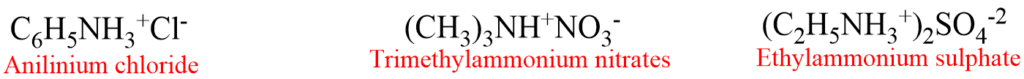
Quaternary ammonium salts may be regarded as derivatives of ammonium salts in which all four H-atoms are replaced by alkyl or aryl groups.
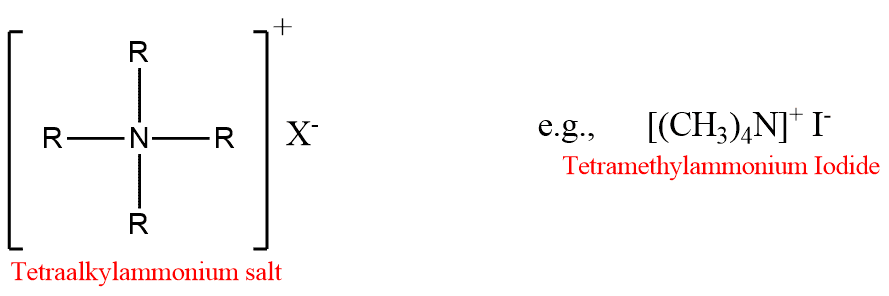
Physical Properties of amines
Physical state and odour
Lower alphabetic amines such as methyl amine and ethyl amine are gases and have an ammonical odour. The higher members are mostly liquids with fishy smells.
Aromatic amines are generally highly toxic and are readily absorbed through the skin. They are colourless when existing in a pure state. But they are easily oxidized by air to form coloured substances.
Boiling points of amines
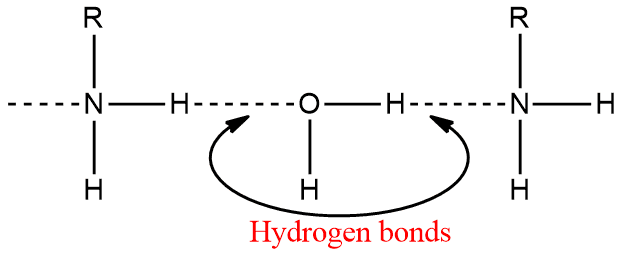
Amines, like ammonia, are polar compounds. Except for tertiary (3o) amines, they can form intermolecular hydrogen bonds.
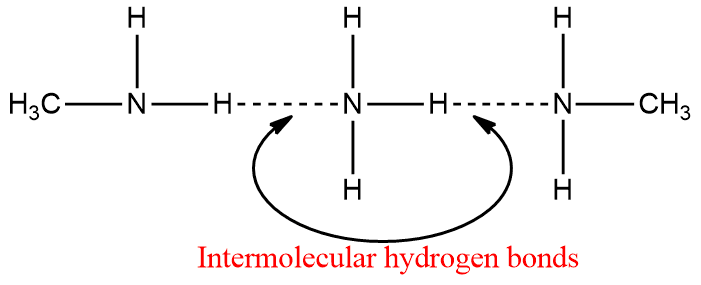
As a result, amines (except 3o amines) have higher boiling points than non-polar compounds of comparable molecular weight. However, they have lower boiling points than alcohols or carboxylic acids. Hydrogen bonding in amines is weaker than that in alcohol and carboxylic acids, and hence the boiling points of amines are lower than those of alcohols and carboxylic acids of comparable molecular mass.
Solubility of amines
Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water. They are also soluble in less polar solvents like ether, alcohol, benzene etc.
Basicity of amines
Due to the presence of lone pairs of electrons on the nitrogen atom of the -NH2 group, the amines are generally basic in nature. They are more basic than water but less basic than hydroxide ions.
The basic strength of amine is expressed in terms of the dissociation constant, Kb. We can compare the basic properties of amines by measuring the extent to which they accept a proton (H+ ion) from water.
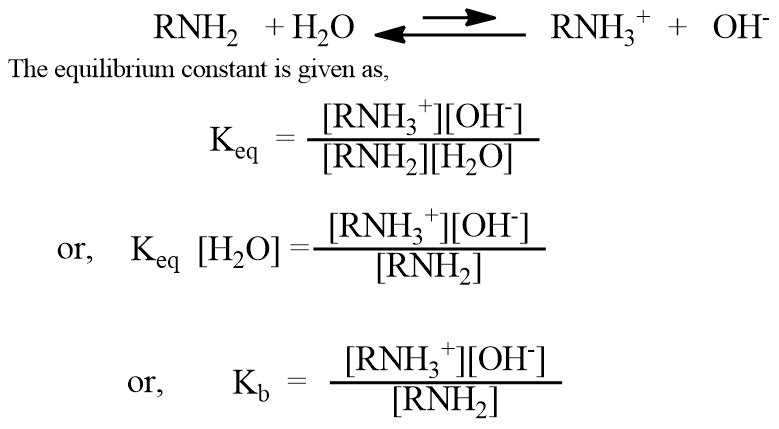
Where Kb = Keq [H2O] is a new constant and is called the dissociation constant or basicity constant of amine, which is a measure of the extent to which the amine accepts H+ ions from water. Each amine has its characteristic Kb. Greater the Kb values, the stronger the base.
Aliphatic amines have Kb values of about 10-3 to 10-4, therefore they are slightly stronger bases than ammonia. But aromatic amines have Kb values of about 10-9 or less, hence they are weaker bases than ammonia. The presence of substituent on the ring also affects the basicity of aromatic amines.
Uses of Amines
- Used in the preparation of azo dyes and other material dyes.
- It leads to the synthesis of amino acids, which are essential for the development of proteins in living organisms.
- Used as painkillers in the form of morphine and Demerol
- They are extensively utilized in the manufacture of compounds for water purification, medicinal, and crop protection.
- Also used as stimulants for neurotransmitters like serotonin in our bodies.
Amines Video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995






