Table of Contents
ToggleWhen a nucleophile attacks hydrogen rather than carbon, elimination reactions occur. A carbon-carbon double bond into a molecule containing only a single bond must necessarily involve the elimination of atoms or groups from two adjacent carbons.
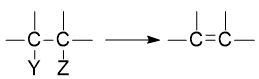
1,2-elimination of HX: Dehydrohalogenation
Dehydrohalogenation involves the elimination of a halogen atom as well as a hydrogen atom from a carbon adjacent with the one losing the halogen. A base is necessary, and its role is to abstract the hydrogen as a proton.
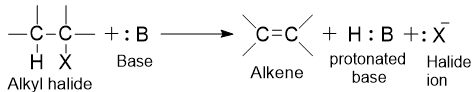
The base :B can be neutral or negatively charged: for example, H2O or OH–.
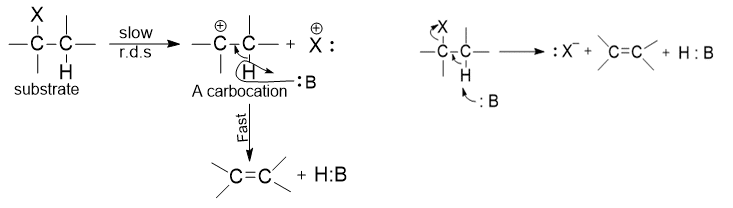
Difference Between E1 and E2 Reaction Mechanism
| E1 reaction mechanism | E2 reaction mechanism |
| E1 reaction is known as elimination unimolecular reaction. | E2 reaction is known as elimination bimolecular reaction. |
| It involves two steps. | It involves a single step. |
| E1 reaction follow first-order kinetics. | E2 reaction follows second-order kinetics. |
| Reactivity of alkyl halide in E1 is 3o >2o >1o | Reactivity of alkyl halide in E2 is 3o >2o >1o |
| Elimination by E1 shows strong saytzeff’s orientation. | show a large hydrogen isotope effect |
| E1 mechanisms are not accompanied by a primary hydrogen isotopes effect | E2 reactions are regioselective and favor the formation of Zaitsev products |
| Rate: K [RX] | Rate: K [RX] [OH–] |
| Example: | Example: |
References
- J. March, Advanced Organic Chemistry, Wiley Eastern Ltd., 1986.
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- Morrison, R. T., & Boyd, R. N., Organic Chemistry, Allyn and Bacon, Inc., 1987.






