Table of Contents
ToggleGouy-Chapman model, also known as the Ionic cloud model, is different from the Helmholtz-Perrin model in various aspects. The Helmholtz-Perrin model fixes the solution charges onto a sheet parallel to the metal, but this model was too rigid to explain the asymmetry of electrocapillary curves and the dependence of capacity upon potential. This may be due to the lack of freedom of the charges in the solution. Let’s see about the Gouy-Chapmann model’s assumption.
Gouy-Chapman Model
In this model, the ions were supposed to be released from a sheet that was parallel to the electrode. Once the ions are free, they become exposed to thermal buffeting from the particles of the solution. The electric force generated by the charges on the electrode, as well as thermal jostling, influence the behavior of ions in the vicinity of the electrode.
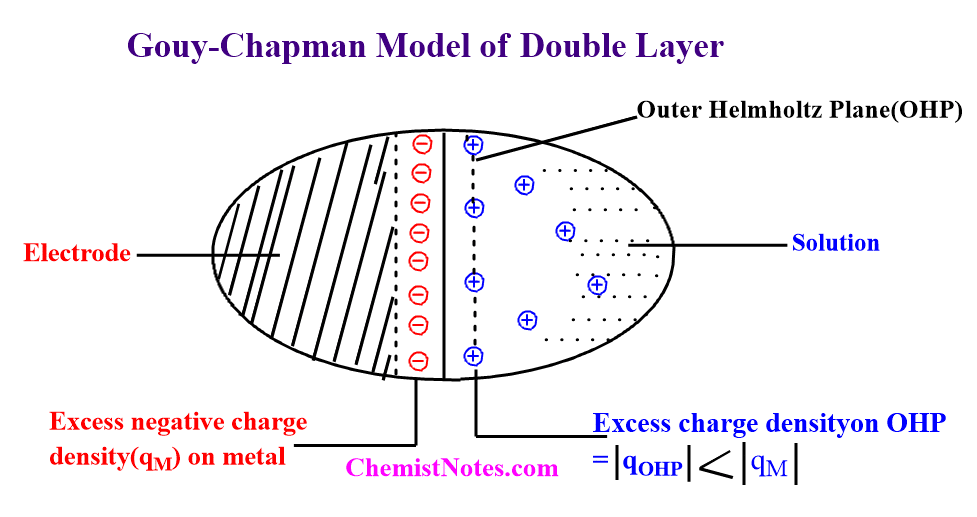
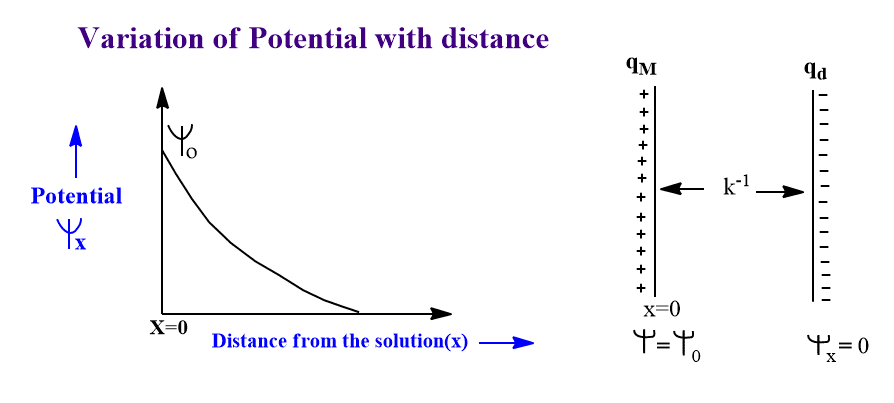
Thus, in the Gouy-Chapmann model, the excess-charge density on the outer Helmholtz plane(OHP) is not equivalent to that on the metal but is less. Some of the solvated ions depart their seats in the second row and travel randomly into the solution. The excess charge density in the solution decreases with distance from the electrode. The electrode has a sort of ionic atmosphere in which, near the metal, its charge attracts the solvated ions to the second row.
Advantages of Gouy-Chapman model over Helmholtz-Perrin Model
The Helmholtz-Perrin model predicts a constant differential capacitance i.e. the capacitance does not change with potential. But this defect has been overcome by the Gouy-Chapman model. According to it, the differential capacitance of an electrified interface is not constant rather inverted parabolic curve is obtained for the capacity-potential curve. This shows the variation of capacitance with potential.
Limitations of Gouy-Chapman model
- The experimental differential capacity-potential curves are just not the inverted parabolas that the G-C diffuse model predicts. The curves are inverted parabolas only in a very dilute solution and at a potential near PZC. But the curves become flattened at a potential far way from PZC.
- The experimental capacity is much lower than the theoretical one at a higher concentration value.
- This theory neglects ion-ion interaction
- The main drawback of this theory was the assumption of the point charge.






