Table of Contents
ToggleClemmensen reduction, examples, mechanisms, and applications in organic chemistry have been discussed here:
Clemmensen Reduction Definition
The reaction involving the reduction of aldehyde or ketone into the corresponding alkane by reaction with amalgamated zinc (Zn-Hg) and concentrated hydrochloric acid is called the Clemmensen reduction reaction. It is applicable to aliphatic, aromatic, alicyclic, and heterocyclic compounds. Some of the examples of Clemmensen reduction involve the reduction of aldehyde or ketone into its corresponding alcohol.
Let’s see an example of Clemmensen reduction reaction
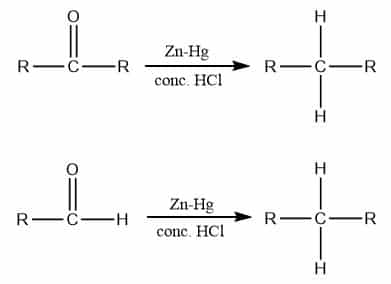
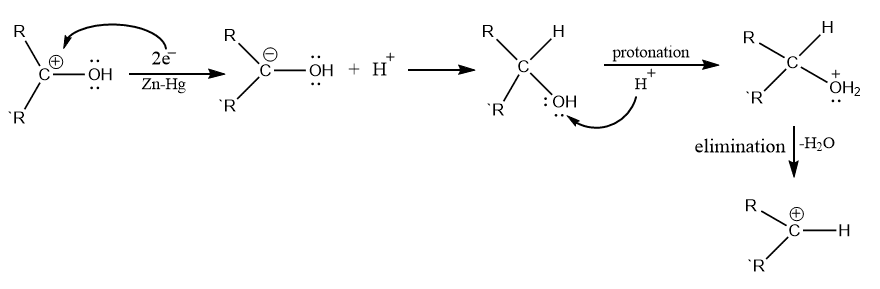
Clemmensen Reduction Mechanism
The Clemmensen reduction mechanism can be carried out either by carbanion mechanism or carbenoid mechanism. The carbanion mechanism involves the direct attack of Zn to protonated carbon, while the carbenoid mechanism is a radical process and involves the reduction at the surface of zinc catalyst.
Let us proceed with the mechanism by carbanion mechanism
Clemmensen reduction entails the transfer of electrons from the catalyst’s metal surface to the protonated carbonyl group’s carbon atom. Four zinc electrons are required for each molecule of aldehyde or ketone. Mineral acid provides a proton to oxygen in this reaction, whereas metal Zn provides an electron pair to an electron-deficient carbonyl carbon to produce carbanion.
The mechanism can be described into 3 following steps:
Step 1: Formation of carbanion
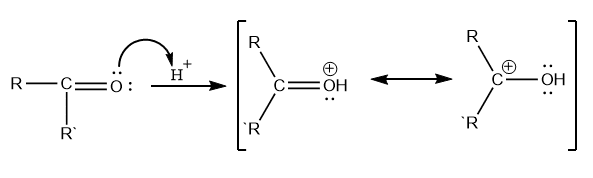
Step 2: Elimination of water
Step 3: Addition of proton
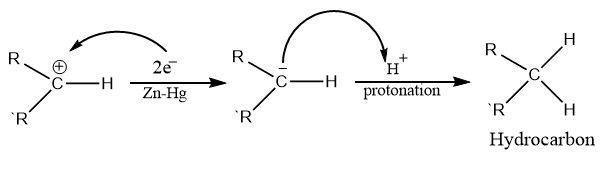
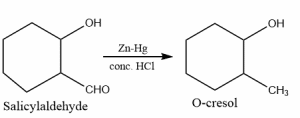
Uses of Clemmensen reduction
- Conversion of salicylaldehyde into O-cresol
2. Conversion of acetophenone into ethylbenzene
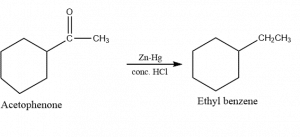
3. Used to convert the carbonyl group into a methyl group
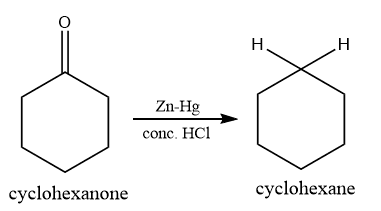
Clemmensen reduction of cyclohexanone
Cyclohexanone can be reduced by Clemmensen reduction into the corresponding alkane via the following way
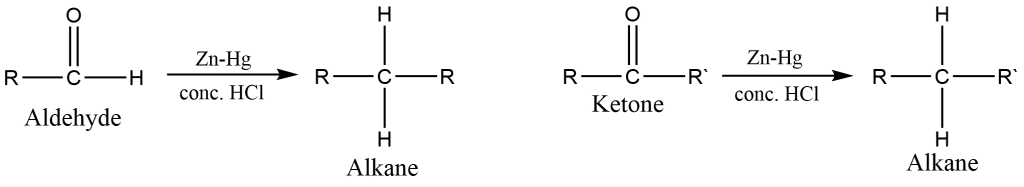
Clemmensen reduction of aldehyde and ketone
The Clemmensen reduction of aldehyde and ketone can be carried out by treating with Zn-Hg and Conc. HCl into following ways:
Difference between wolff kishner and clemmensen reduction
| Wolf Kishner reduction | Clemmensen reduction |
| The reaction that involves the reduction of hydrazone, semicarbazone or azines of aldehydes and ketones to hydrocarbons under basic conditions is known as wolf-kishner reduction | The reaction involving the reduction of aldehyde or ketone into the corresponding alkane by reaction with amalgamated zinc (Zn-Hg) and concentrated hydrochloric acid is called the Clemmensen reduction reaction. |
| Reduction of aldehyde or ketone into methylene group. | Reduction of aldehyde or ketone into the corresponding alkane. |
| It requires strong basic condition | It requires a strong acidic condition |
| No catalyst is required | Zn-Hg catalyst is used during the reaction |
| Such reaction is not sensitive to base-sensitive materials/substrate. | Such reaction is not sensitive to acid-sensitive materials/substrate |
Clemmensen reduction limitations
Some of the limitations of Clemmensen reduction are:
- The reaction is incompatible with acid-sensitive materials.
- This approach also fails to reduce the -COOH group.
FAQs/MCQs
Clemmensen reduction reagent
Amalgamated zinc (Zn-Hg) and conc. hydrochloric acid (HCl) is the Clemmensen reduction reagent.
Clemmensen reduction conditions
As Clemmensen Reduction allows the deoxygenation of aldehydes or ketones, the substrate must be stable enough against strong acid and the conditions must be strongly basic.
Clemmensen reduction of benzaldehyde
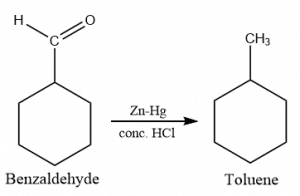
Clemmensen reduction of benzaldehyde gives toluene
Why amalgamated zinc (Zn-Hg) catalyst is used in the Clemmensen reduction?
Since catalyst involves the transfer of electrons from its metal surface to the protonated carbonyl group’s carbon atom to produce carbanion, it is highly used. Four zinc electrons are required for each molecule of aldehyde or ketone.
What is Clemmensen reduction?
The reaction involving the reduction of aldehyde or ketone into the corresponding alkane when treated with Zn-Hg and Conc. HCl called as Clemmensen reduction
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.







One Response
I really appreciate your teamwork. I’m happy to see such a topic in easy method. best of luck to all.