Table of Contents
ToggleWestern blotting, called immunoblotting, is a scientific method for identifying particular proteins in a sample. It entails the size-based separation of proteins using gel electrophoresis, the transfer of the proteins onto a membrane, the identification of the target protein using specialized antibodies, and finally the detection of the protein.
Definition of Western blotting
Western blotting, often known as the protein immunoblot or western blot, is a common analytical technique in molecular biology and immunogenetics for identifying specific proteins in tissue homogenates or extract samples. This method is used not only to identify the proteins but also to visualize, separate, and measure the various proteins in a complex protein combination.
History of Western Blotting
W. Neal Burnette described the process of Western blotting in a paper that was published 31 years ago. The study was initially turned down by the publication Analytical Biochemistry, but it quickly gained popularity among molecular biologists as a preprint. Ultimately, the journal published the paper, which has since received more than 6,000 citations.
Burnette didn’t put his name on the blot, so it’s possible that the current generation of researchers is unaware that he was engaged in creating the technology that is now widely used in molecular biology and biochemistry research laboratories and employed as a clinical diagnostic for HIV-AIDS.
Principle of Western blotting
The concepts of equal protein loading, protein separation by molecular weight, electrophoretic transfer to a suitable membrane, and antibody probing are the foundation of the Western blot. For downstream analysis, proper sample preparation for upcoming electrophoresis is essential.
Western Blotting Protocol
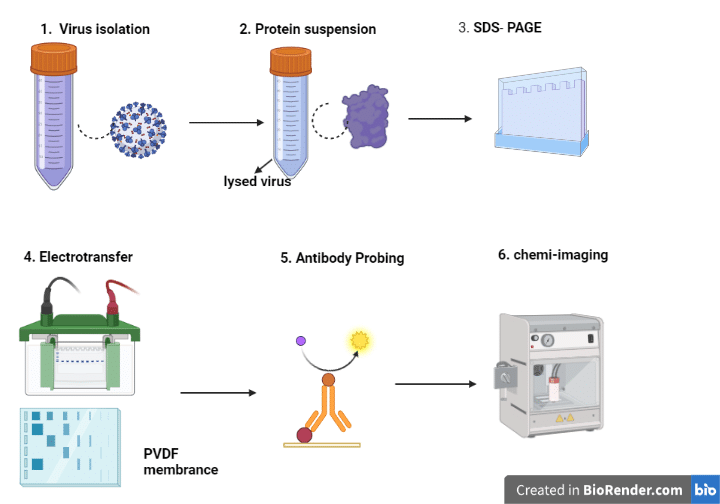
- Sample Preparation: Using a lysis buffer, the proteins are separated from the biological sample, such as cells or tissues. By using a protein test, such as the Bradford assay, the protein concentration is evaluated.
- Gel electrophoresis: Polyacrylamide gel electrophoresis (PAGE) divides the proteins into different sizes. Acrylamide, a catalyst, and a cross-linking agent are combined to form the gel. The protein sample is mixed with a loading buffer containing a reducing agent and then heated to denature the proteins. By utilizing an electrophoresis device, the samples are placed into the gel’s wells and the gel is run.
- Transfer: Using a transfer apparatus, the separated proteins from the gel are transferred onto a membrane, such as nitrocellulose or PVDF membrane. To stop non-specific antibody binding, the membrane is then blocked with a blocking solution, such as 5% non-fat dried milk in TBST (Tris-buffered saline with 0.1% Tween-20).
- Primary antibody incubation: By using primary antibodies that are made to recognize the desired target protein(s) to incubate the membrane. The membrane is incubated with diluted primary antibodies in a blocking solution for an overnight period at 4°C with gentle shaking.
- Secondary antibody incubation: To eliminate any unattached primary antibodies, the membrane is washed with TBST before incubating it with a secondary antibody coupled to a detection molecule, such as horseradish peroxidase (HRP). The membrane is incubated for 1-2 hours at room temperature with gentle shaking after the secondary antibody has been diluted in a blocking solution.
- Detection: A detection molecule, such as an enhanced chemiluminescence substrate (ECL), is used to identify the target protein(s) after washing the membrane with TBST to remove any unbound secondary antibodies. Autoradiography film or a chemiluminescence imaging device is used to see the signal.
Elisa Vs Western Blotting
The immunosorbent assay known as ELISA is used to find antibodies or antigens in samples. Proteins can be isolated and identified from mixtures using the analytical process known as western blotting and ELISA does not need the gel electrophoresis step but western blotting needs the gel electrophoresis step.
Western blot is a method that detects a specific protein from a protein mixture, whereas ELISA is a method that detects the presence of antigens and antibodies in the patient’s blood. The main distinction between Elisa and Western Blot is this.
Advantages of Western blot over ELISA
Western blotting is less sensitive than ELISA, which makes it more challenging to find low-abundance proteins. provides more details about the protein: Using a variety of variables, including protein size, a Western blot can verify that the right protein is being tested.
Western Blotting Ladder
StrepTactin-HRP Conjugate proteins (or Strep-tagged recombinant proteins) are mixed in Western blot protein ladders, which are pre-stained. It enables western blot immunodetection using chemiluminescence or colorimetry and fluorescent protein gel visualization.
Western blotting Application
After cloning, the production of proteins is continually monitored using a western blot. Additionally, it is used in medical diagnostics, such as the BSE-Test and HIV test. A western blot is used in the confirmatory HIV test to find anti-HIV antibodies in a sample of human serum.
How western blotting differs from other Southern and northern blotting?
In molecular biology, there are three different methods for detecting particular molecules, such as proteins, RNA, and DNA: western blotting, northern blotting, and southern blotting. The type of molecule being detected and the method of detection used varied significantly between different techniques.
Protein blotting, another name for western blotting, is a technique for identifying certain proteins in a complicated mixture. Prior to being deposited onto a nitrocellulose or PVDF membrane, the proteins are first sorted by size using gel electrophoresis. Next, a secondary antibody that is conjugated to a detection enzyme, such as horseradish peroxidase (HRP), is applied to the membrane to probe it with a primary antibody specific to the protein of interest.
Specific RNA molecules are found using the Northern blotting method. Gel electrophoresis is used to split RNA samples into different sizes, after which they are transported to a membrane, usually made of nylon. After that, a labeled DNA probe complementary to the target RNA is used to probe the membrane. When the probe binds to the RNA on the membrane, the RNA can be seen.
To find certain DNA molecules, southern blotting is employed. Prior to utilizing gel electrophoresis to segregate the DNA samples by size, the samples are first digested with a restriction enzyme. On a nylon membrane, the DNA fragments are transferred before being probed with a labeled DNA probe that is complementary to the DNA of interest. The probe binds to the DNA on the membrane, enabling visualization of the DNA.
In conclusion, southern blotting finds DNA, northern blotting finds RNA, and western blotting finds proteins. Each methodology has a unique strategy for detection and separation.