Table of Contents
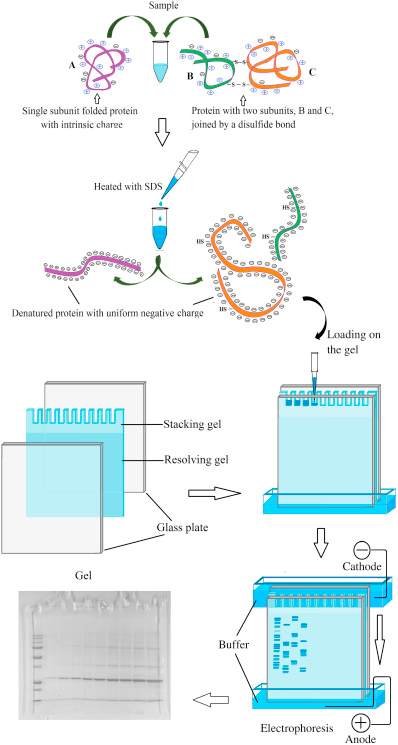
ToggleSDS PAGE stands for sodium dodecyl-sulfate polyacrylamide gel electrophoresis. Proteins are divided in polyacrylamide gel according to their molecular weight in the SDS PAGE. It is an extremely effective method for studying proteins. The protein samples are first prepared by being mixed with a loading buffer that contains SDS, mercaptoethanol, bromophenol blue, and glycerol.
Prokaryotic and eukaryotic cell, tissue, viral, and environmental samples can all be biologically used to create protein samples. The sample is heated to 950C for 5 minutes before adding the sample buffers. Large biomolecules containing many amino acids make up proteins. Organic compounds known as amino acids comprise an amine, a carbonyl group, and a side organic chain.
SDS PAGE definition
SDS PAGE is a technology which is used in order to separate proteins with molecular weights between 5 and 250 kDa, Ulrich K. Laemmli created the discontinuous electrophoretic technology. Protein may be separated by mass using the electrophoresis technique known as SDS-PAGE. The medium is a discontinuous gel made of polyacrylamide, often known as the “matrix”. In a slab gel, the polyacrylamide-gel is normally encased between two glass plates.
Principle of SDS PAGE
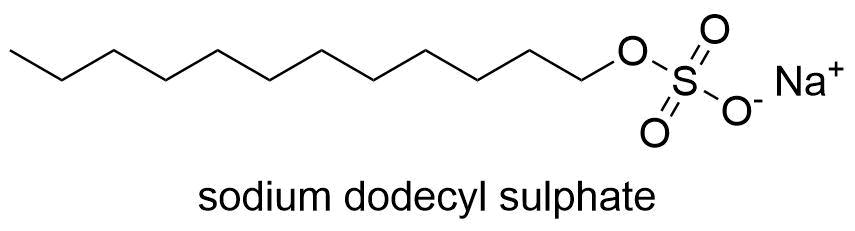
SDS has a polar head group with a lengthy hydrophobic chain and a net negative charge. It has sulfuric acid bonds. When a protein is heated, mercaptoethanol is used as a reducing agent to break the sulfide bond. When SDS binds to a protein evenly, the protein’s intrinsic charge is significantly reduced in comparison to the negative charge added by SDS. This moves the folded protein closer to the negatively charged linear molecule. Therefore, this protein can be separated in polyacrylamide gel based on its molecular weight.
Protocol of SDS PAGE technique
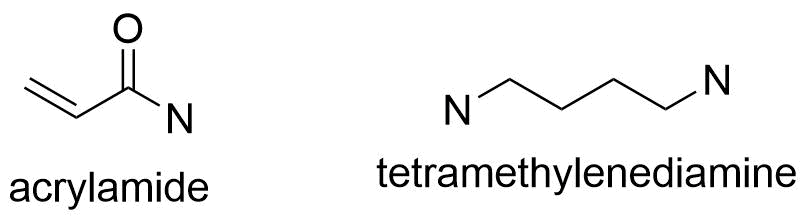
- Acrylamide and tetramethylenediamine (TEMED) are the two main ingredients of the separating gel, which has a pH of 8.8, and the stacking gel, which has a pH of 6.8.
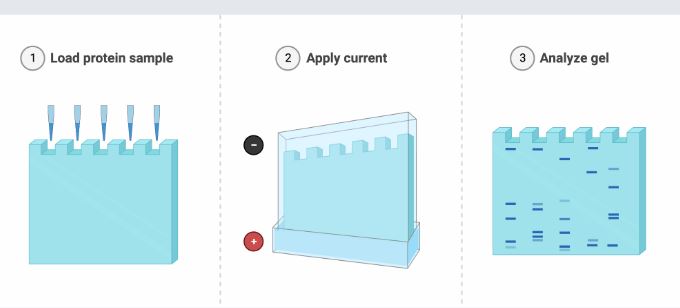
- The gel is created by polymerizing the two gel plates that have been fixed vertically. The solid separating gel is covered with the stacking gel.
- A small well can form on the gel due to the plate that is positioned on top of the stacking gel. The main difference between stacking gel and separating gel is that stacking gel is of high porosity and buffered with Tris-Cl buffer at pH 6.8 whereas separating gel contains a high percentage of acrylamide and is cast in Tris-Cl buffer at PH 8.8.
- After the gel has polymerized, the coil is taken out and the gel is applied to the electrode. The negative electrode is placed on top of the gel, while the positive electrode is positioned at the bottom.
- Tris-glycine chloride buffer with a pH of 6.8 is poured after the gel is placed within the chamber to enable the current to flow through the gel.
- To ensure that the protein remains in the running buffer after it has been denatured, SDS is also included in the gel and running buffer.
Some chemical and its uses in SDS PAGE
- Tris-HCL
- To assist in dragging the proteins across the gel, hydrochloric acid, often known as HCl, is added to the loading buffer, typically in the form of Tris-HCl. The Cl ions pass across the gel more quickly than the proteins because of their negative charge.
- SDS
- In the SDS-PAGE sample buffer, there is a detergent called SDS. Proteins’ disulphide bonds are broken by SDS and certain reducing chemicals, which causes the tertiary structure of the proteins to be disrupted.
- glycerol
- Glycerol gives the sample density, which helps it sink to the bottom of the loading wells and prevents it from evaporating out of the well while the remaining gel is being loaded. A dye called bromophenol blue makes it easier to see the materials in the wells and how they travel across the gel.
- bromophenol dye
- The sample contains the indicator dye bromophenol blue. Its major objective is to demonstrate how the proteins move across the gel over time. It moves through the gel in the same direction as the proteins due to its small negative charge.
- mercaptoethanol
- Oxygen radical levels are decreased by the reducing agent and antioxidant beta mercaptoethanol (BME). It is often applied at a concentration of 5 % to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). BME denatures proteins by cleaving intermolecular disulfide bonds, which causes this.
Application of SDS PAGE
- It is very useful for the test of purity of proteins.
- It is useful in the determination of molecular weight of proteins.
- It helps in characterizing the size and different types of oligomeric proteins.
- It is useful in Western Blotting and protein ubiquitination (a tightly regulated, highly specific, and ATP-dependent biological process carried out by a complex cascade of enzymes).
- It is useful in HIV tests to separate the HIV proteins.
- This technique is suitable for separating low molecular weight molecules.
- In addition to the sample, a molecular weight size marker is placed into the gel for sample application. This contains proteins with established sizes, which aid in estimating the size of proteins in real samples of samples that travel parallel through the gel.
- Glycerol increases the density of the sample while bromophenol dye is employed to make it visible. An electric field is then used.
- Negatively charged proteins move from a negatively charged electrode to a positively charged electrode as the electric field is applied, moving toward the gel. We can observe that tiny proteins move through the gel with ease while large proteins move more slowly.
Limitation of SDS PAGE
- It is an expensive technique so it is difficult to afford.
- It intentionally denaturants proteins before electrophoresis.
- Proteins isolated by SDS-PAGE typically cannot be tested for enzymatic activity, protein binding interactions, or protein cofactor detection.