Table of Contents
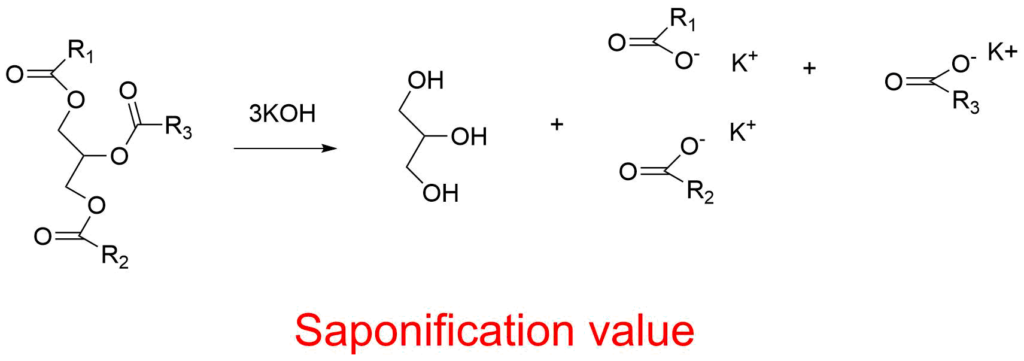
ToggleSaponification value is the amount of sodium hydroxide (NaOH) or potassium hydroxide (KOH) needed to saponify one gram of fat under the given circumstances (SV or SN). It is a measurement of the overall sample’s triglyceride-containing fatty acids’ average molecular weight (or chain length). Making soap is just the process of saponification. Basically, soaps are merely long-chain fatty acid salts of potassium or sodium. In the process of saponification, an ester and an inorganic base combine to create soap and alcohol.
Saponification value definition
The saponification value is the mass in mg of potassium hydroxide (KOH, sometimes referred to as potash), which is required to neutralize the free fatty acids and saponify the esters present in one gram of material. The number of mg of potassium hydroxide needed to completely neutralize the free and combined acids contained in 1 g of a fuel sample is known as the saponification number of the fuel.
Saponification value principle
The amount of potassium hydroxide (KOH) needed to thoroughly hydrolyze (saponify) one gram of oil or fat is known as the saponification value. In actual fact, an amount of oil or fat known to be present is refluxed with an excess of a standard alcoholic potash solution, and the unused alkali is titrated against a standard acid.
Formula to calculate Saponification value
The formula to calculate saponification value are:
Saponification Value = (A – B) x N x 56.1/W
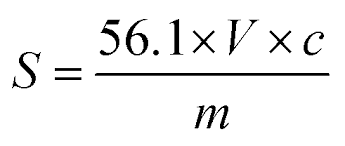
- A= H2SO4, for blank, mL
- B= H2SO4, for sample, mL
- W= weight of sample (dry basis), g
- N= normality H2SO4 solution
- 56.1= equivalent weight of potassium hydroxide
Reagents required
- Potassium hydroxide, 0.5 N solution in ethyl alcohol, standardized to ± 0.005
- Hydrochloric acid, 0.5 N solution, standardized
to ± 0.005. - Phenolphthalein indicator, 1% (visual titration
only).
Protocol to estimate SV.
- 2 g of sample are weighed into a 300-mL Erlenmeyer flask to the closest 0.01 g.
- 25 mL of 0.5 N ethanolic potassium hydroxide is poured into a pipet and is mixed for 60 minutes.
- Titration is carried out using an automated titrator or a phenolphthalein indicator at a temperature of 60 to 70 degree C with 0.5 N hydrochloric acid.
- The same procedure is also used to run a blank.
Saponification value of some oils
| Oils | SV |
| almond butter | 90-140 |
| almond oil | 180-195 |
| castor oil | 188-203 |
| coconut oil | 135.3 |
| acai oil | 188-203 |
| olive oil | 135.3 |
| palm oil | 142 |
Comparison between saponification, acid, and iodine value
The main distinction between acid value and saponification value is that the former provides the mass of potassium hydroxide needed to neutralize one gram of a material while the latter provides the mass needed to saponify one gram of fat. And The amount of unsaturation in a fat or oil is indicated by the iodine value (IV). It is described as the quantity of iodine that is absorbed by 100 grams of fat.
Highest SV value
Due to the high lauric and myristic acid content of some vegetable oils, such as coconut and palm kernel oils, their saponification values are much higher (235-260 mg KOH/g oil).
References
https://www.sciencedirect.com/topics/engineering/saponification-value