Table of Contents
ToggleAcid value (AV), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. It’s the amount of base typically potassium hydroxide (KOH) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.
What is Acid value?
The acid value is a metric for the quantity of carboxylic acid groups (C(=O)OH) in a chemical compound, such a fatty acid, or in a blend of chemical compounds. It is a measurement of the amount of free fatty acids (FFAs) in a material. A common approach involves dissolving a known quantity of sample in an organic solvent (typically isopropanol) and titrating it with a solution of alcoholic potassium hydroxide (KOH) at a known concentration using phenolphthalein as a color indicator.
Principle of Acid value
The oil or fat in an alcoholic media is directly titrated against a standard potassium hydroxide or sodium hydroxide solution to ascertain its acid value.
RCOOH + KOH = RCOO– K+ + H2O
Chemical required
- Balance machine
- burrete
- hot plate
- measuring cyclinder
- conical flask
Solution required
- NaOH / KOH
- ethanol
- Phenolphthalein indicator
Procedure to Estimate acid value
Following are the procedure to estimate the acid value of given sample:
- First of all 0.1 g- 0.3 g of fat sample is taken in 100 mL of Erlenmeyer flask.
- And 10 mL of n-hexane is added and along with 1-2 drops of indicator.
- Then the solution is titrated against 0.02 N KOH solution. The end point is reached when pink colour persist for 30 seconds.
- The blank test is also carry out without taking fat sample.
- Then calculation is carried out.
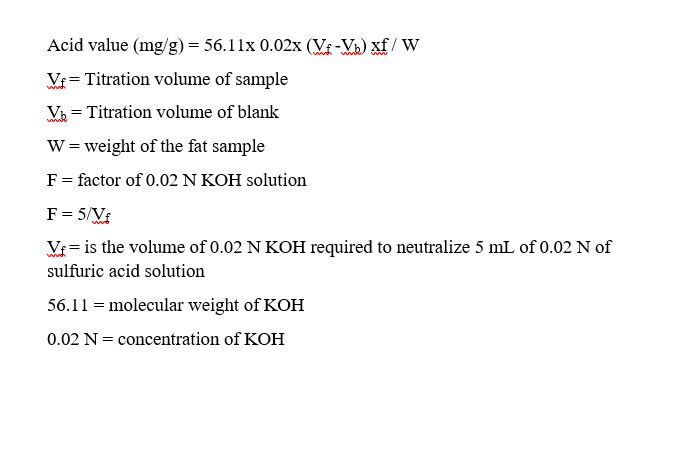
Formula to calculate acid value
Reliable application
- The amount of rancidity of the provided fat is indicated by the acid value. Rancified oils provide high acid levels. Rancidification is the breakdown of fats and other lipids by oxidation and/or hydrolysis.
- Acid Value is a crucial sign of the quality of vegetable oil. The amount of potassium hydroxide (KOH, in milligrams) required to neutralize the free fatty acids present in 1 g of oil is the acid value.
Iodine value Vs Acid value
The amount of iodine in grams that is consumed by 100 grams of a chemical compound is known as the iodine value (IV; often referred to as the iodine absorption value, iodine number, or iodine index) in chemistry. When assessing the level of unsaturation in fats, oils, and waxes, iodine values are frequently utilized. Unsaturation in fatty acids mostly manifests as double bonds that are highly reactive to halogens, in this example, iodine.
Acid value (AV), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. It’s the amount of base—typically potassium hydroxide (KOH)—expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.
Acid value of some oils
The acid value of some oils :
| S.N | Oil | Acid value |
| 1 | maize | 0.223 |
| 2 | soya | 0.60 |
| 3 | virgin oil | 6.6 |
| 4 | used frying oil | 31 |
| 5 | canola | 0.071 |
| 6 | bee’s wax | 17-36 |
What does the acid value indicates?
The amount of potassium hydroxide milligrams needed to neutralize the free fatty acids found in one gram of fat is known as the acid value. Given that free fatty acids are often produced during the breakdown of triglycerides, it is a relative indicator of rancidity.
What does high acid value indicates?
The quality of the oil decreases with increasing acid value and free fatty acid level. As triglycerides break down with time into fatty acids and glycerol, the acid value of an oil also rises with age.
FAQs/MCQs
Which has lowest acid value?
Canola has lowest acid value.
Which has highest acid value?
The highest acid value is of virgin olive oil.
What is acid value?
The amount of potassium hydroxide needed to neutralize the free fatty acids in 1.0 g of fat or oil is known as the acid value.