Table of Contents
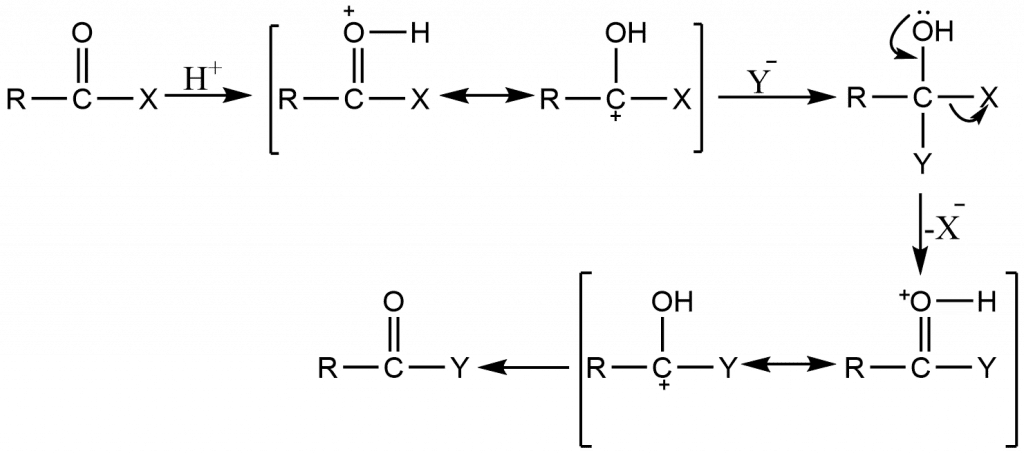
ToggleFor aliphatic trigonal carbons, nucleophilic substitution is very important, especially when the carbon is doubly bonded to oxygen, sulfur, or nitrogen. As predicted, acid catalyzes this reaction because protonation reduces the electron density near the carbon undergoing substitution, which facilitates the attack of nucleophiles.
The tetrahedral process, also known as addition-elimination, occurs with much less ease than with carbonyl groups because the negative charge of the intermediate must be carried by carbon, which is less electronegative than oxygen, sulfur, or nitrogen:
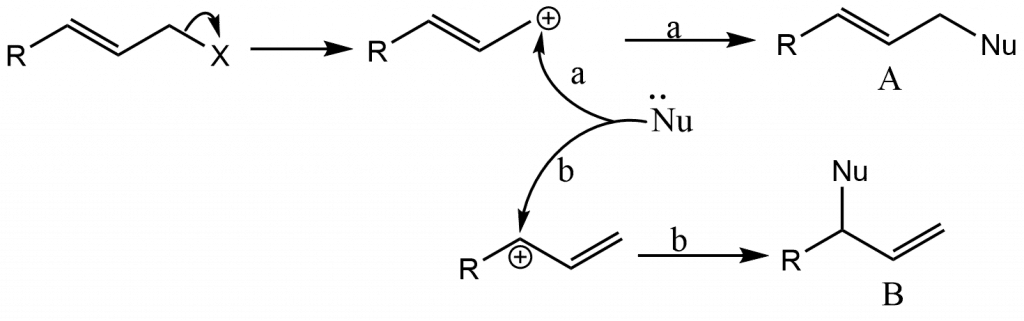
Nucleophilic Substitution at an Allylic Carbon: Allylic Rearrangements
Allylic substrates undergo nucleophilic substitution reactions quickly, but we treat them separately because they are frequently accompanied by a type of rearrangement known as allylic rearrangement. When allylic substrates are treated with nucleophiles under SN1 conditions, two products are often obtained: the regular product and a rearrangement product.
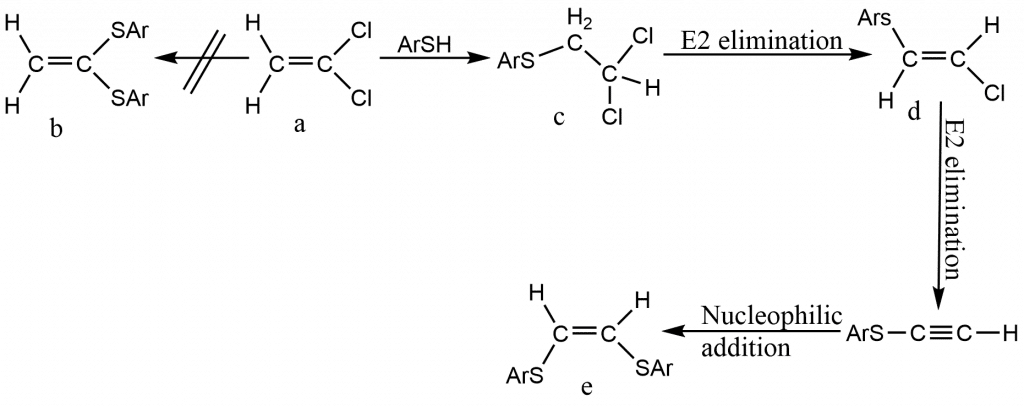
Nucleophilic substitution at vinylic carbons
Although nucleophilic substitution at a vinylic carbon is challenging, there are several occurrences. The tetrahedral process and the closely related addition–elimination mechanism are the most prevalent mechanisms. At a saturated substrate, both of these processes are impossible. The addition–elimination mechanism for the reaction between 1,1-dichloroethene (a) and ArS catalyzed by OEt has been shown. The result was the “rearranged” chemical (e), not the 1,1-dithiophenoxy compound (b). The isolation of (c)and (d) revealed that an addition-elimination process had occurred. ArSH adds to the double bond (nucleophilic addition) in the first step, yielding the saturated (c). The alkene (d) is produced in the second stage by an E2 elimination process. The result of a second elimination and addition is (e).
Refrences
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987






