Table of Contents
ToggleBefore explaining the Bronsted Bjerrum equation, It is necessary to know the background of the salt effect and solution kinetics because it explains how the rate of reaction fluctuates with the addition of electrolytes.
The kinetic study of the chemical reactions in the solution phase is known as solution kinetics and the nature of the solvent present greatly affects the kinetics of such reactions. Not only solvents but the presence of non-reacting or inert ionic species in solution can also change the rate of reaction as reported in the literature. Such an effect of charged species on the kinetics of the reaction is termed a salt effect.
kinetic salt effect
The effects of ionic strength on ionic reaction rate is known as a kinetic salt effect. The Bronsted Bjerrum equation best explains the kinetic salt effect.
The rate of reaction can be influenced by the ionic strength, which is the measurement of the total ion concentration in a solution. We know factors like temperature, pH, and catalysts affect the rate of reaction but ionic strength plays an important role. It can increase or decrease the rate of reaction depending on the kind of reaction.
Primary salt effect and secondary salt effect are considered two major types of kinetic salt effect on the basis of the effect of charged species.
What is Primary kinetic salt effect?
If the concentration of electrolyte affects the activity coefficient and consequently the rate of reaction, such an effect is known as the primary kinetic salt effect.
What is secondary kinetic salt effect?
If the addition of electrolytes changes the actual concentration of reacting ions, then such an effect is known as a secondary kinetic salt effect.
Bronsted Bjerrum equation derivation
Let A and B be reactants which react as follows.
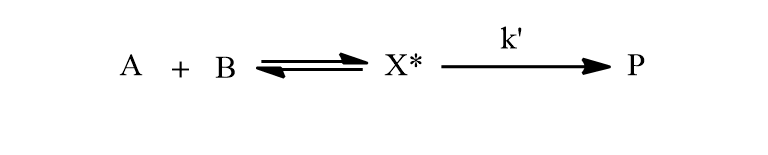
where X* is the intermediate species and is in equilibrium with reactants A and B. Therefore,
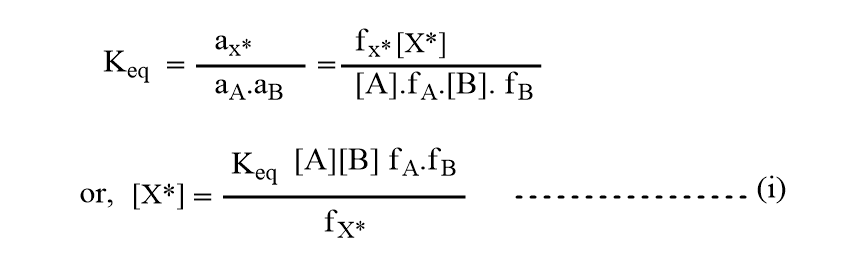
According to Bronsted-Bjerrum, the rate of reaction can be given as,
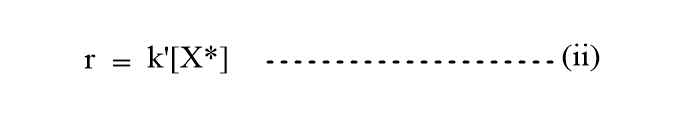
Introducing the value of [X*], then the rate will be,
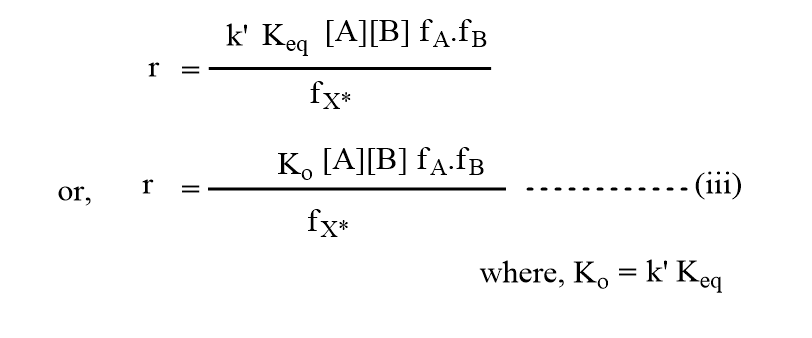
Again, the experimental rate will be,
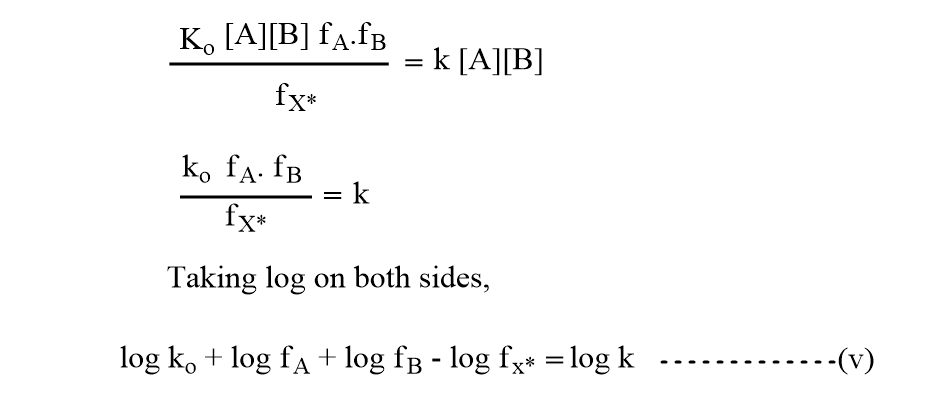
Equations (iii) and (iv) should be equal as both represent the same reaction rate.
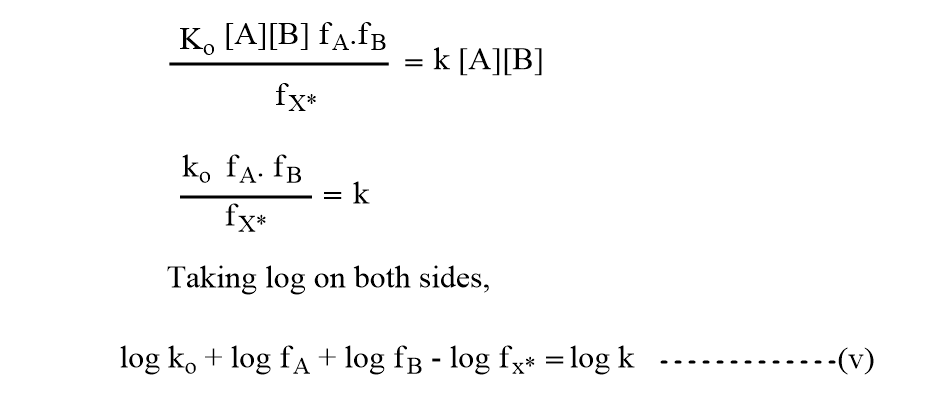
Debye-Huckel limiting law relates the activity coefficient with ionic strength as,
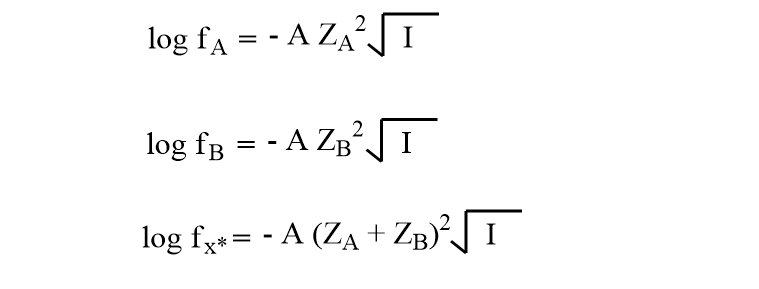
Using these values in equation (v),
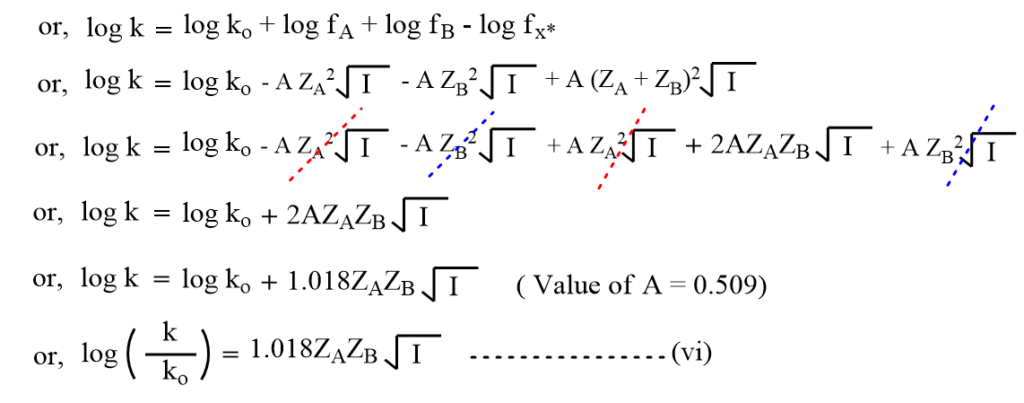
where,
k= rate constant at given ionic strength
ko=rate constant at zero ionic strength
ZA and ZB are the total charges on reactants A and B respectively.
I= ionic strength of the solution.
This equation (vi) is known as the Bronsted-Bjerrum equation, which gives the effect of ionic strength on the reaction rate.
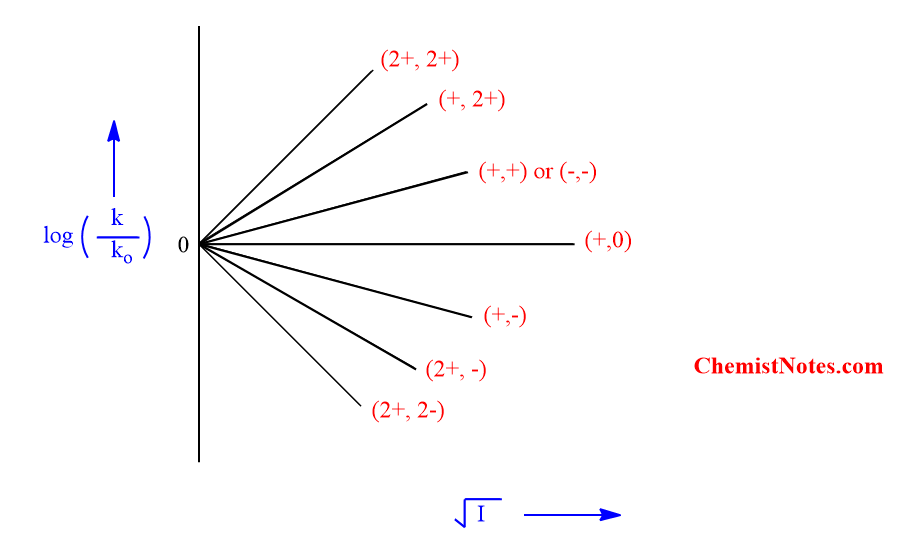
This equation (vi) predicts that the plot of log(k/ko) versus (I)1/2 should be a straight line and for an aqueous solution, the slope is nearly equal to the product of the ionic charges ZAZB.
Three special cases of Bronsted-Bjerrum equation
Case i: If ZA and ZB have the same sign then ZAZB is positive and the rate constant increases with ionic strength.
Case ii: If ZA and ZB have the opposite sign then ZAZB is negative and the rate constant decreases with ionic strength.
Case iii: If one of the reactants is unchanged then ZAZB is zero and the rate constant is independent of the ionic strength.
FAQs/MCQs:
What is ionic strength?
Ionic strength measures the total concentration of ions in a solution and can affect the reaction rate.
What is kinetic salt effect?
The effects of ionic strength on ionic reaction rate is known as a kinetic salt effect.