Table of Contents
ToggleThe glyoxylate cycle, a modified form of the tricarboxylic acid cycle, is a metabolic pathway that takes place in plants, protists, bacteria, and fungi. The glyoxylate cycle is primarily focused on the enzymatic conversion of acetyl-CoA into succinate, which serves as a precursor for the biosynthesis of carbohydrates. In context to the plant cells, it occurs in glyoxysomes. This cycle is mostly active in germinating plant seeds and in a small number of bacteria that rely only on acetate for carbon.
Vertebrates are unable to perform gluconeogenesis from acetate or fatty acids due to the absence of the Glyoxylate cycle.
Glyoxylate Cycle
In plants, certain invertebrates, and in some microorganisms like E. coli and yeast, acetate can serve both as an energy-rich fuel and as a source of phosphoenol pyruvate for carbohydrate synthesis. In these organisms, a glyoxylate cycle occurs which allows the net conversion of acetate into oxaloacetate. A certain enzyme of the citric acid cycle operates in two modes in such organisms:
- They can function in a citric acid cycle for the oxidation of acetyl-CoA to CO2 as it occurs in most tissues, and
- They can operate as part of a specialized modification, the glyoxylate cycle.
The glyoxylate cycle may have been involved before and give rise to the citric acid cycle. The overall reaction equation of the glyoxylate cycle (also regarded as an anaplerotic pathway) is:

Glyoxylate cycle steps
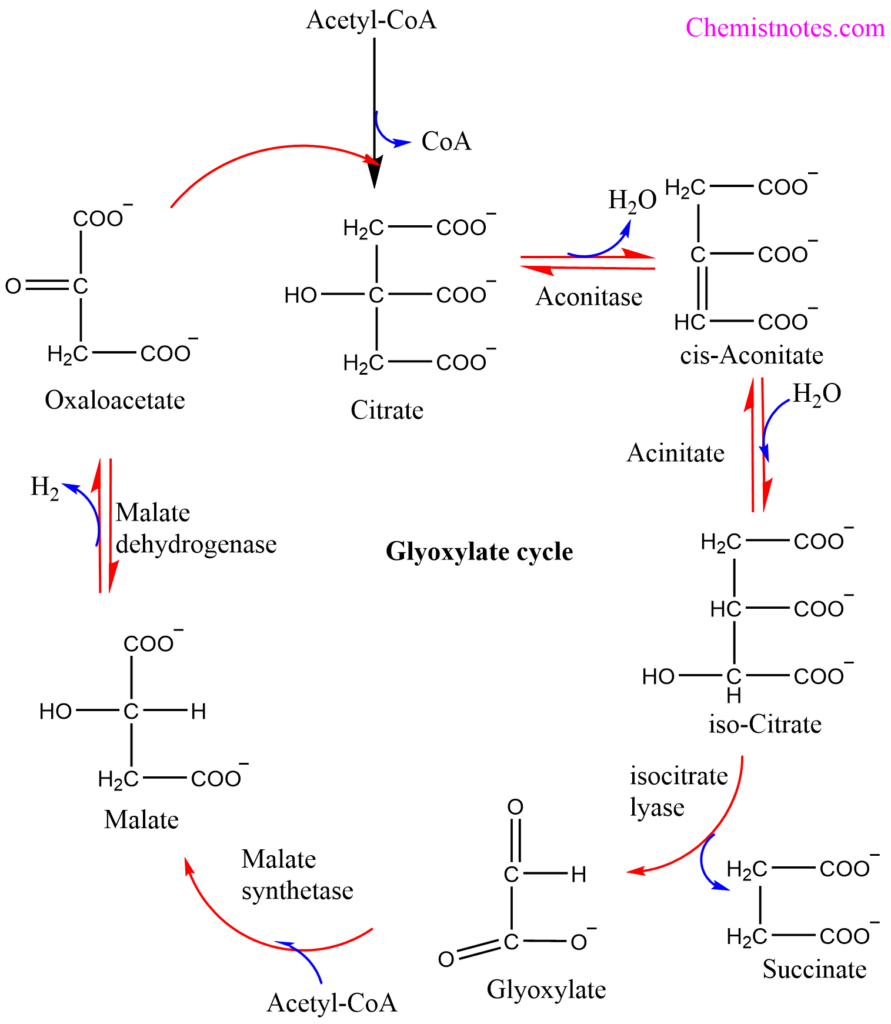
Step 1: Generally, in the glyoxylate cycle, acetyl-CoA condenses with oxaloacetate to form citrate exactly as in the citric acid cycle.
Step 2: In the second step citrate forms a cis-aconitate with the release of one molecule of water and the reaction is catalyzed by an aconitase enzyme, similar to that of a citric acid cycle.
Step 3: Cis-aconitate is then catalytically hydrolyzed to form isocitrate. This step is catalyzed by the aconitase enzyme.
Step 4: In this reaction, the breakdown of isocitrate does not occur via isocitrate dehydrogenation reaction, however, it occurs through a cleavage reaction forming succinate and glyoxylate. The reaction is catalyzed by the isocitrate lyase enzyme.
Step 5: Thus, formed glyoxylate condenses with acetyl-CoA to yield malate and the reaction is catalyzed by malate synthetase enzyme.
Step 6: The malate is then subsequently oxidized to oxaloacetate, the oxaloacetate can condense with another molecule of acetyl-CoA to repeat the cycle.
In each turn of the glyoxylate cycle, two molecules of acetyl-CoA enter and there is a net synthesis of one molecule of succinate, available for biosynthetic purposes. The succinate can be converted through fumarate and malate into oxaloacetate, which can then be converted into phosphoenol pyruvate by the PEP carboxykinase enzyme. Phosphoenol pyruvate can serve as a precursor of glucose via gluconeogenesis.
In plants, the enzymes of the glyoxylate cycle are sequestered in membrane-bounded organelles called glyoxysomes; those enzymes common to the citric acid and glyoxylate cycles have two isozymes, one specific to mitochondria and other to the glyoxysome. Glyoxysomes are not present in all tissues at all times. They develop in lipid-rich seeds during germination, before the developing plants acquire the ability to make glucose by photosynthesis.
Glyoxylate cycle vs TCA Cycle
The Glyoxylate cycle involves a collection of eight enzymes, five of which are interconnected with the Tricarboxylic Acid (TCA) cycle. These enzymes include citrate synthase, aconitase, succinate dehydrogenase, fumarase, and malate dehydrogenase. The glyoxylate cycle and the conventional tricarboxylic acid (TCA) cycle exhibit differences in the conversion of isocitrate. Specifically, in the glyoxylate cycle, isocitrate is transformed into glyoxylate and succinate through the enzymatic action of isocitrate lyase (ICL), as opposed to its conversion into α-ketoglutarate in the TCA cycle.