Table of Contents
ToggleWet chemical synthesis of nanoparticles is one of the most attractive, simplest, and versatile bottom-up methods. It is more advantages than the sol-gel process since alkoxides utilized in wet chemical synthesis are more versatile and economical than inorganic salt compounds used in the sol-gel technique. Nanoparticles are generated from a homogenous solution by one of the following approaches:
- Precipitation from a supersaturated solution
- Chemical reduction
- Ultrasonic decomposition
Precipitation method for synthesis of nanoparticles
Metal oxide nanoparticles can be produced by altering the pH of the precursor solution. To synthesize CuO nanoparticles, for example, add NaOH to a solution of Cu(NO3)2.3H2O or CuCl2 until the final pH is 14. When NaOH is added to the solution of these salts while it is being vigorously stirred, a dark CuO solid precipitate forms, and when dried, it produces nanostructured CuO particles.
Chemical reduction method for synthesis of nanoparticles
Metals are reduced to a zero-valent state, followed by nucleation and growth, to produce metallic nanoparticles when metallic salt solutions are treated with the appropriate reducing agents. One of the main difficulties is preventing the agglomeration of nanoparticles produced by chemical reduction. By forming layers of charged molecules on the nanoparticle surface, the use of surfactants or capping agents during reduction helps in dispersing nanoparticles and prevents them from aggregating.
For example, by reducing HAuCl4 using reducing agents such as sodium citrates, ascorbic acid, sodium hydroxide, etc., one can produce gold nanoparticles. Some reducing substances, such as sodium citrate, coat the surface of gold nanoparticles, preventing the aggregation of the particles due to electrostatic attraction. As a result, producing nano-dispersed gold nanoparticles using citrate synthesis is quite reliable.
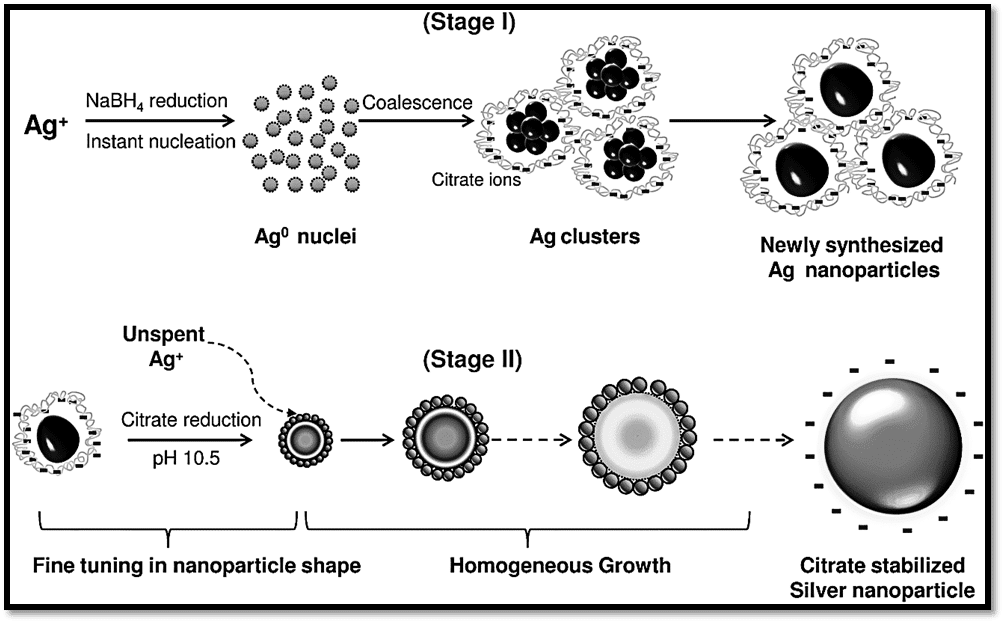
Similar to gold nanoparticles, silver nanoparticles can also be prepared by adding reducing agents to suitable silver salts. Variation of the protocol can help in the synthesis of different sizes of nanoparticles.
A simple schematic of synthetic steps of silver nanoparticles, that involves the reduction of silver salts by sodium borohydride is represented below:
Ultrasonication method nanoparticles
The use of high-intensity ultrasound offers a simple and adaptable way for the synthesis of certain nanoparticles that is not usually possible by other techniques. With a very quick heating and cooling cycle of 1010 K/S, ultrasonication offers unique reaction conditions of extremely high temperature (5000 K) and pressure (1000 bar) in a small volume (bubbles) for a very short period of time.
Two significant physical phenomena associated with the formation of nanoparticles by ultrasonication methods are cavitation and nebulization. The formation, growth, and implosive collapse of bubbles in a liquid is referred to as cavitation or acoustic cavitation. The severe circumstances created inside the collapsing bubble are what cause a number of sonochemical processes in liquids or liquid-solid slurries. Nebulization is the term used to describe the process of producing mist using ultrasound that penetrates a liquid and impinges at the liquid-gas interface.
Generally, nanoparticles synthesized by sonochemical methods are spherical in shape and other shapes such as triangles, cubes, squares, etc. are not feasible by this method. However, under special reaction conditions and in presence of other chemical components, the synthesis of different shapes has been also reported.
Sonochemical or ultrasonic synthesis of noble metal nanoparticles has been used for the synthesis of various nanoparticles such as Au, Ag, Pt, Pd, etc. Some of the advantages include:
- Reding agents are not required
- Fast reaction rates
- Synthesis of small-sized nanoparticles
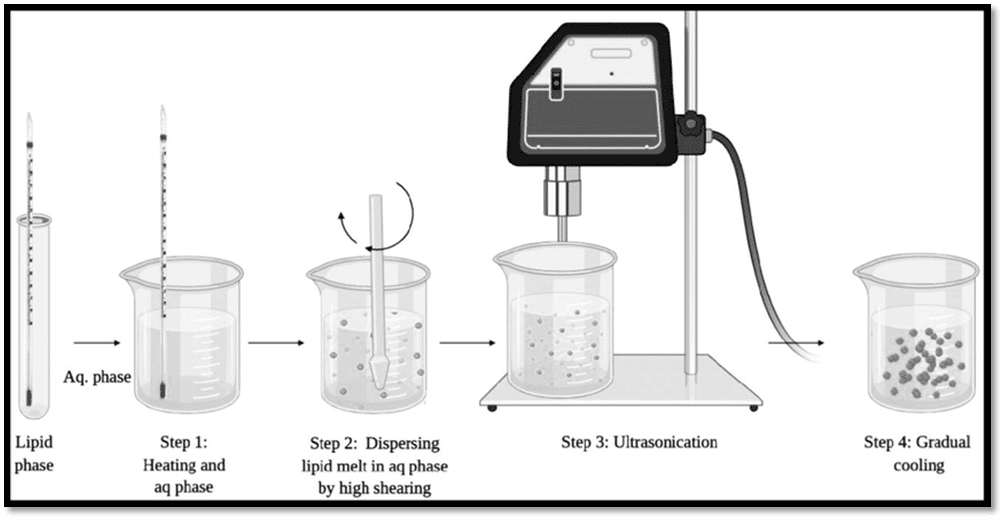
In contrast to sonochemistry, which is mediated by direct chemical reactions caused by sonic waves, ultrasonic pyrolysis involves thermally driven nanoparticle synthesis. Ultrasound assists in the phase separation of individual microdroplets in this technique. Ultrasonic pyrolysis employs high-frequency, low-intensity ultrasounds such that l Low-intensity, high-frequency ultrasound waves nebulize a solution to produce phase-separated micron-sized particles. This process involves heating liquid droplets produced by nebulization in the gas phase, which results in a solid or liquid phase reaction.
Advantages of wet chemical synthesis
- Simple and economical methods for the synthesis of nanoparticles
- High purity
- Uniform shapes and sizes of synthesized nanoparticles
Disadvantages of wet chemical synthesis
- Requirement of high reaction temperature
- Use of surfactants
- large-scale production is challenging.
Wet chemical synthesis video
References
- S. Agnihotria, S. Mukherjiabc and S. Mukherji, Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy, RSC Adv., 2014, 4, 3974-3983.
- Yasir Beeran Pottathara, Yves Grohens, Vanja Kokol, Nandakumar Kalarikkal, Sabu Thomas, Chapter 1 – Synthesis and Processing of Emerging Two-Dimensional Nanomaterials, Nanomaterials Synthesis, Elsevier, 2019, Pages 1-25, ISBN 9780128157510, https://doi.org/10.1016/B978-0-12-815751-0.00001-8.