Table of Contents
ToggleSelf assembly of nanoparticles refers to the organization of molecules or particles into well-defined aggregates or structures without any external intervention. Moreover, Nanoparticle self assembly is a significant bottom-up synthetic methodology that involves the aggregation of colloidal nanoparticles into the final desired structure. This aggregation may occur chemically as a result of the complementary binding of organic molecules and supramolecules (molecular self-assembly), or it may occur spontaneously (entropically) and as a result of thermodynamic minima (energy minimization) restrictions.
One of the most crucial methods utilized in biology for the development of complex functional structures is molecular self-assembly. Self-assembly is a potent bottom-up assembly and manufacturing technique since it is not restricted to molecules or the nano-domain and can be done on almost any scale (multi-scale ordering). The ability to combine the self-assembly characteristics of organic molecules with the electrical, magnetic, and photonic properties of inorganic components is another appealing aspect of this method.
Self assembly principle
The principle behind the self assembly process is the spontaneous (physical) or chemical aggregation of colloidal nanoparticles. The process of spontaneous self-assembly takes advantage of the ability of monodispersed nano- or submicro-colloidal spheres to organize into a face-centered cubic (FCC) lattice.
The system’s goal to reach a thermodynamically stable state is the driving force behind this process. Other than naturally occurring thermal self-assembly, complex 3D structures can also be created by applying gravitational, convective, and electro-hydrodynamic forces.
Chemical self-assembly needs the attachment of a single molecule organic layer (self-assembled monolayer, or SAM) to the colloidal particles (organic or inorganic), and the subsequent self-assembly of these components into a complex structure via molecular recognition and binding.
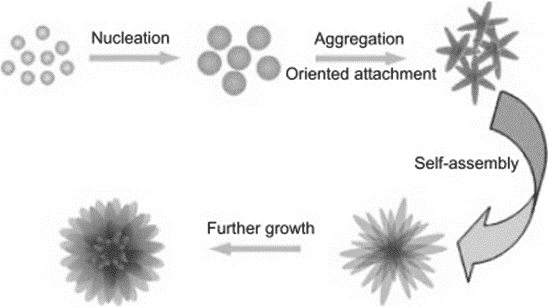
Physical self assembly
In physical self assembly, colloidal nanoparticles aggregate to form different structures driven by thermodynamic energy minimization. Different shapes and sizes of structures are obtained due to the balance of electrostatic forces, surface tensions, entropic factors, substrate topography and affinity, size shape, and concentration of particles.
The size and shape of the nanoparticles are determined during self-assembly, which is initiated by evaporation, drying kinetics, substrate roughness, surface wetting/dewetting, hydrodynamic processes, and self-diffusion on the substrate. It is highly challenging to regulate the shape and size of nanoparticles by self-assembly since self-assembly uses very small forces.
Other methods, such as template-assisted and electrical and magnetic force-driven procedures, are widely utilized in addition to spontaneous self-assembly to produce 2D to 3D-assembled structures of nanoparticles. Colloidal particles are trapped by the limited regions of templates in a template- or pattern-guided technique, resulting in the formation of various forms that are dictated by the template structure.
Chemical self assembly
A crucial element in the self-assembly of colloidal particles is provided by organic and supramolecular (SAM) molecules. SAMs are solid substrates that are chemically adsorbed with strong organic molecules. They typically have a long, hydrophobic (non-polar) tail that stretches outward and a hydrophilic (polar) head that can attach to various solid surfaces. When a substrate is submerged in a diluted solution of the molecule in an organic solvent, SAMs are produced. A dense collection of molecules arranged to expose the end group is the resulting film.
The success of the attachment to the substrate’s surface has a significant impact on a SAM’s durability. Because the end group may be functionalized to produce precisely ordered molecular arrays for a variety of applications, from straightforward, ultra-thin insulators and lubricants to intricate biological sensors, SAMs have received a lot of attention.
Chemical self-assembly uses organic or supramolecular SAMs as the binding and recognition sites for fabricating complex 3D structures from colloidal nanoparticles. Some commonly used organic monolayers are:
- Organosilicon compounds on glass and native surface oxide layer of silicon
- Alkanethiols, dialkyl disulfides, and dialkyl sulfides on gold
- Fatty acids on alumina and other metal oxides
- DNA
The most popular organosilane utilized in the formation of SAMs is octadecyl trichlorosilane (OTS), primarily because it is simple, easily accessible, and produces excellent, dense layers. This method’s drawback is that a cloudy coating is produced on the surface as a result of the production of a gel of polymeric siloxane when the alkyl trichlorosilane in the solvent adhering to the substrate is exposed to water. Another important organic SAM system is the alkanethiols X(CH2)nSH, where X is the
end group) on gold. The fact that gold does not have a stable oxide and may therefore be handled under ambient circumstances is a significant benefit of utilizing gold as the substrate material.
Soft lithography is another technique for depositing alkanethiol SAM. This method is based on applying alkanethiol ink to a PDMS stamp before transferring it to substrates that are both planar and nonplanar. Planar, non-planar, and spherical alkanethiol functionalized surfaces can also be utilized to self-assemble a range of complex 3D structures.
Moreover, the building block of all life, deoxyribonucleic acid (DNA), can be utilized to self-assemble nanomaterials into useful macroscopic aggregates with a variety of desirable physical properties.
Advantages of self assembly
- Simple
- Inexpensive
- Versatile method
- Capable of producing 2D nanostructured metal oxides, such as nanosheets
- Applicable in drug delivery
- Application in biosensors, biomedical science, imaging techniques, photocatalysis, and so on.
Self-assembly video
References
- Bangwei Zhang, Chapter 5 – Principles, Methods, Formation Mechanisms, and Structures of Nanomaterials Prepared via Self-Assembly, In Micro and Nano Technologies, Physical Fundamentals of Nanomaterials, William Andrew Publishing, 2018, Pages 177-210, ISBN 9780124104174, https://doi.org/10.1016/B978-0-12-410417-4.00005-8.
- S. K. Ghosh, A. Böker; Self-Assembly of Nanoparticles in 2D and 3D: Recent Advances and Future Trends, Wiley Online Library, 14 August 2019. https://doi.org/10.1002/macp.201900196