Table of Contents
ToggleSol gel methods are based on the principle of transition of materials from the solution or sol phase to the gelatinous or gel phase. It is a process of oxide network formation via polycondensation reaction in the solution phase. Sol–gel process, one of the bottom-up approaches, has been in use for many years for producing metal oxide and ceramic powders with high purity and high homogeneity. Moreover, this method is suitable for the synthesis of composite nanopowder, the generation of oxide nanoparticles, and the production of inorganic-organic hybrid materials
Sol-gel method of preparation of nanomaterials
In sol-gel method, hydrolysis of reactive metal precursors occurs, followed by condensation and polymerization reactions. In general, when a liquid alkoxide, water, a co-solvent, and an acid or base as a catalyst are combined, M-O-M crosslinks (oxo-bridges) are created by removing an alkoxide’s alkyl groups. These crosslinks then condense to create a three-dimensional solid-phase network.
Initially, metal oxides such as methoxides (M-OCH3) and ethoxide (M-OC2H5) undergo hydrolysis where the alkoxy group OR is replaced by hydroxo ligands (OH). This step is called ligand exchange or hydrolysis.
M(OR)n + H2O → M(OH)(OR)n–1 + ROH
where R is an alkyl group, CnH2n+1. The reaction mechanism of this step involves the addition of a
negatively charged HOδ– group to the positively charged metal center (Mδ+), followed by the elimination of R-OH. Some of the factors like nature of the solvent, nature of the alkyl group, concentration of each species in the solvent, temperature, water to alkoxide molar ratio, and presence of acid or base as catalyst affect hydrolysis reactions.
Following condensation, either alcohol or water is eliminated to produce metal oxide or hydroxide linkages. Condensation only occurs when the cation M is bound to at least one hydroxo ligand, which is referred to as M-OH for convenience. Moreover, condensation results from the reactions of olation and oxolation. While oxolation includes the formation of oxo bridges (M-O-M) between two metal cations, olation is a reaction in which the hydroxo or “ol” bridge (M-OH-M) between two cations is formed:
Olation: M – OH + M – OH2 → M – OH – M + H2O
Oxolation: M – OH + H – OM → M – O – M + H2O
M – OH + ROM → M – O – M + ROH
Condensed oxide or hydroxide species are produced when two metal atoms form “ol” or “oxo” bridges between them. Extended linear M-O-M chain polymers grow into three-dimensional solid phase networks in acid conditions. The acid-catalyzed polymerization is believed to occur in three stages:
- Polymerization of monomer units to form particles
- Growth of particles
- Formation of chains or networks that extend throughout the liquid medium which thickens to form a gel.
The sol-to-gel transition in acid solution enables the solid phase to be molded into films, fibers, and monoliths. In contrast, a colloidal sol is produced upon basic hydrolysis of metal alkoxides. When the solid network is composed of rounded sol particles, the gel is colloidal.
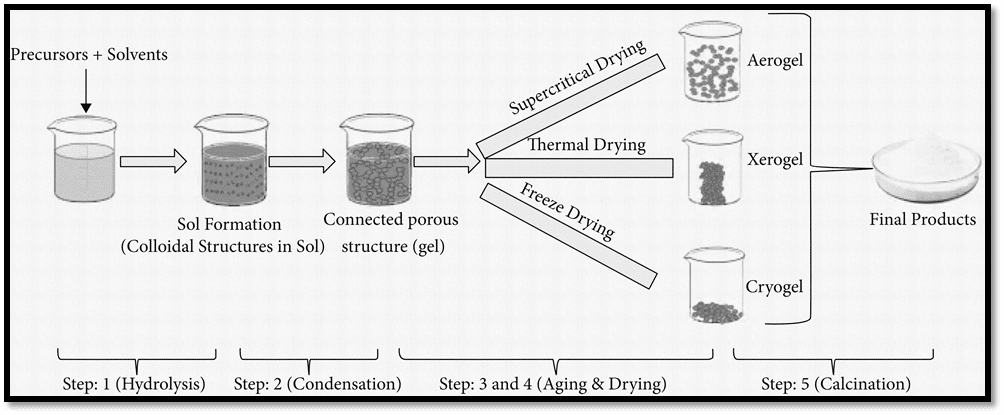
In order to form a solid structure, gelatinous material is further processed, and solvents are eliminated. Solvent that has been trapped in a porous structure or between two networks is released either by drying at room temperature or under supercritical conditions. Gels obtained by the removal of solvent at ambient condition called xerogels, while the gels prepared under supercritical condition is called aerogel.
Xerogels lead to significant shrinkage and breaks in the gel structure. The very strong interfacial tension created at the vapor-liquid interface of the microscopic pores causes this structure to collapse. Meanwhile, aerogel retains the original structure with very little shrinkage.
Sol Gel process
Since silicon tetraethyl orthosilicate [TEOS; Si(OC2H5)4] is the most common alkoxide, it undergoes partial or complete hydrolysis forming silanol.
Si(OR)4 + H2O → Si(OR)3(OH) + ROH
Since silicon alkoxides hydrolyze relatively slowly, adding acid or base catalysts speeds up the process of turning metal precursor molecules into trialkoxy silanol, Si(OR)3(OH). The amount of water and catalyst present will determine whether hydrolysis proceeds partially, converting metal alkoxides into Si(OR)4n(OH)n, or proceeds fully, converting all OR groups in alkoxides to OH:
Si(OR)4 + 4H2O → Si(OH)4 + 4ROH
Further condensation yields cluster species Si-O-Si (Siloxane)
(OR)3Si – OH + HO – Si(OR)3 → (OR)3Si – O – Si(OR)3 + H2O
(OR)3 Si – OR + HO – Si (OR)3 → (OR)3Si – O – Si(OR)3 + ROH
In short, the sol gel method can be illustrated as
Advantages of Sol Gel Method
The sol–gel process has been useful for synthesizing only metal oxides as a result of the
presence of metal–oxygen bonds in the alkoxide precursor, and the resulting gels are essentially
metal hydroxides or oxides. When it comes to producing metal oxide nanoparticles, this method has several significant advantages over others. Among them are the production of high-purity powders as a result of the homogenous molecular mixing of the raw components and the large-scale industrial manufacturing of nanopowders. Besides these, some of the major advantages are:
- Synthesis of metal oxide nanoparticles
- Cost-effectiveness and high chemical product composition control are two benefits of the sol-gel method.
- Synthesis products with high purity
- Ability to produce thin layers of amorphous materials using this approach
- Surface coverage
- production of geometrically shaped optical components
- The sol gel method can be utilized as a molding substance in the production of ceramics and as a transitional layer between thin films of metal oxides in a variety of applications.
Disadvantages of Sol Gel Method
The disadvantage of Sol gel method is the high cost of alkoxide precursors.
Sol Gel method Video
References
- D. Bokov, A. Turki Jalil, S. Chupradit, et al., “Nanomaterial by Sol-Gel Method: Synthesis and Application”, Advances in Materials Science and Engineering, 2021. doi: https://doi.org/10.1155/2021/5102014
- G. V. Aguilar, “Introductory Chapter: A Brief Semblance of the Sol-Gel Method in Research”, December 12th, 2018. DOI: 10.5772/intechopen.82487