Table of Contents
ToggleSpray pyrolysis is one of the most commonly used aerosol processing bottom-up methods that involves the atomization of chemical precursors into aerosol droplets that are dispersed throughout a gas medium. On transporting aerosols in a hot reactor, the solution gets evaporated and forms ultrafine particles or thin films. Several particles, including multi-component compounds, have been synthesized by using this method. Compared to gas-to-particle conversion techniques, it is a more simple and more economical technique.
In spray pyrolysis, an aqueous solution is atomized in a number of reactors where the aerosol droplets go through evaporation and solute condensation; drying and thermolysis are then followed by sintering. Nanoparticles can be produced directly, through the synthesis of droplets, or by releasing the particular crystallites that make up the spray pyrolysis-derived particles from the thermolysis stage.
Spray pyrolysis can be used to prepare several nanoscale metal oxides like Al2O3, ZnO, ZrO2, etc.
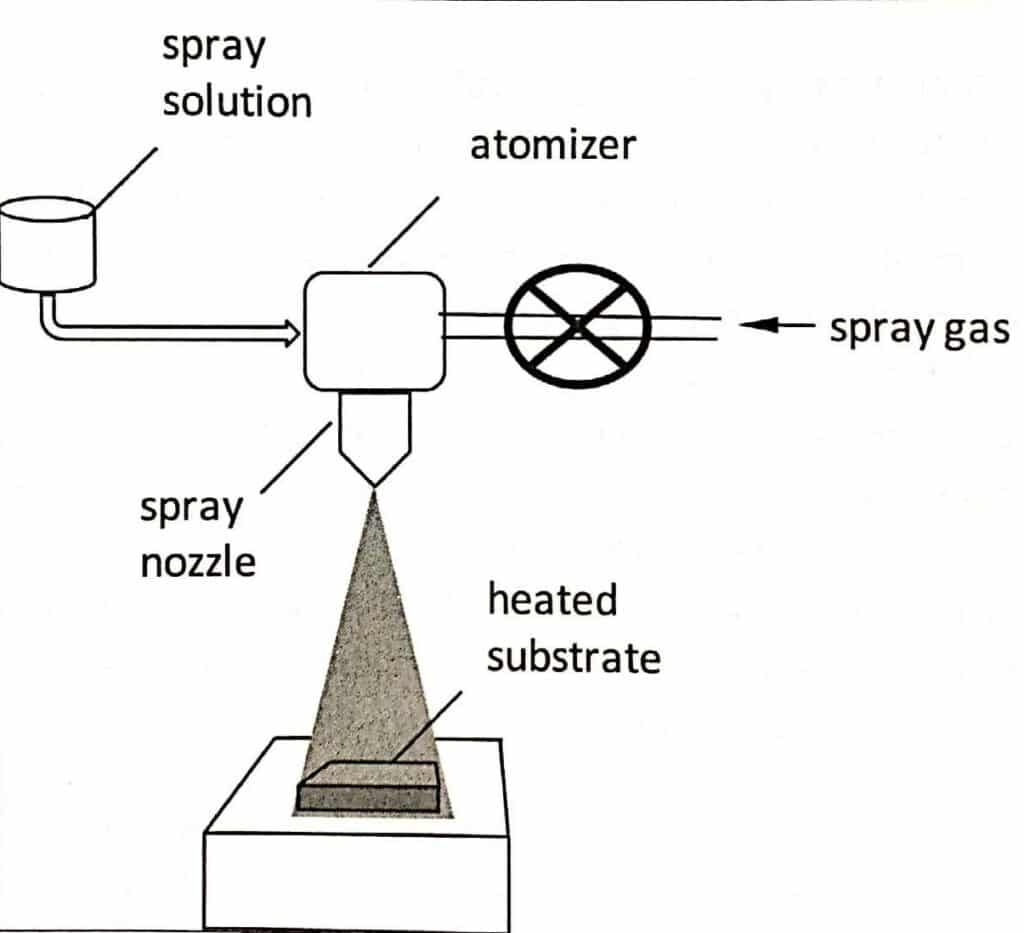
Spray pyrolysis method for nanoparticles
A typical spray pyrolysis process consists of the following steps and associated components:
- Preparation of solution
- Conversion of the starting solution into an aerosol droplet by an atomizer or nebulizer
- Transportation by the carrier gas
- Pyrolysis of the aerosol droplet on a heated substrate to get nanoparticles or thin films.
Solution preparation, the initial stage of spray pyrolysis, involves dissolving metallic salt in a suitable solvent. The preparation of tiny droplets of the solution by forcing them through a nozzle is the second phase, also known as droplet formation or atomization. Droplets are then introduced onto a heated surface in the following phase. The solvent is evaporated lastly, followed by thermolysis and sintering to produce nanoparticles.
Flame spray pyrolysis
Flame spray pyrolysis involves dissolving or mixing nanoparticle precursors with flammable liquids or solvents, such as toluene, ethanol, etc., and spraying the mixture into the flame zone (combustion flame). Depending on the experimental conditions, the spray goes through combustion and the precursors are transformed into metal oxide or nanosized metal. Wide varieties of inorganic nanoparticles such as spinels (generally represented by MAl2O4, where M is a metal other than Al), metal oxide nanopowder like TiO2 and Al2O3, and even metal alloys can be generated by a flame pyrolytic method. The major advantages of this process are low cost and high production rates.
Advantages of spray pyrolysis method
- Open atmosphere process
- Ability to observe the deposition process in progress
- Continuous and affordable operation
- High yield
- Not necessary to use high-quality regents as precursors.
- High surface area allows for adjustment of crystal size
- The products comprise homogeneous compositions
Disadvantages of spray pyrolysis method
- The powder materials’ yield is extremely low.
- It is necessary to convert the sulfides into oxides.
- Various challenges with regard to growth temperature optimization.
Spray Pyrolysis Video
References
- Malik, O., Hidalga-Wade, F. J. D. L., & Amador, R. R. (2017). Spray Pyrolysis Processing for Optoelectronic Applications. Pyrolysis. doi: 10.5772/67431
- Falcony C, Aguilar-Frutis MA, García-Hipólito M. Spray Pyrolysis Technique; High-K Dielectric Films and Luminescent Materials: A Review. Micromachines (Basel). 2018 Aug 19;9(8):414. doi: 10.3390/mi9080414. PMID: 30424347; PMCID: PMC6187587.