Table of Contents
ToggleAnti-infective agents definition
Anti-infective agents or anti-infective drugs are those compounds that are used to kill or prevent the growth of microorganisms responsible for various diseases or simply these are agents which prevent the transmission of infection by the destruction of pathogenic microorganisms.
Antiseptic compounds vs Disinfectant
Antiseptic compounds kill or prevent the growth of microorganisms when applied to living tissue while a disinfectant prevents transmission of infections by the destruction of pathogenic microorganisms when applied to inanimate objects( not living objects).
What is selective toxicity?
Selective toxicity is the property of certain drugs to kill one type of organism while not harming others.
Anti-infective agents classification
Anti-infective agents can be classified according to a different scheme such as chemical type of compounds, biological property, or therapeutic indication.
On the basis of the biological properties or therapeutic indication, anti-infectives are classified into the following types.
- Antibacterial agents
- Antivirals agents
- Antimalarial agents
- Antiprotozoal agents
- Antifungal agents
- Anthelmintics agents.
Moreover, there are numerous classes of chemical compounds capable of showing local anti-infective properties. These groups are listed below.
- Alcohols and Aldehydes
- Phenol derivatives
- Oxidizing agents
- Halogen containing compounds
- Dyes
- Mercury compounds(Mercurials)
- Preservatives
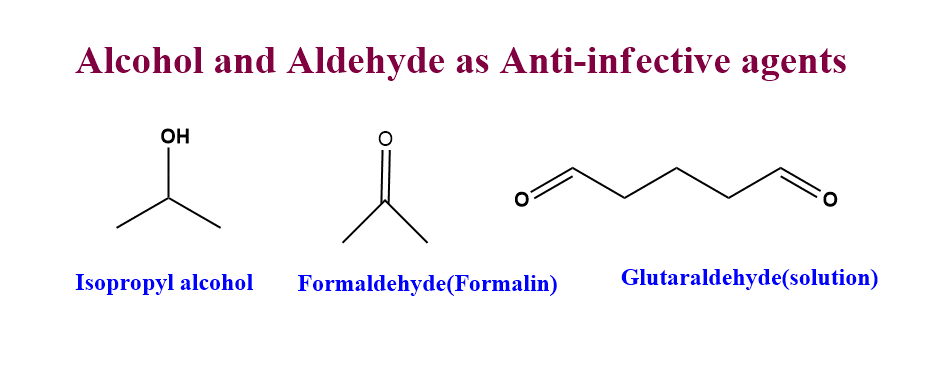
Alcohols and aldehydes as anti-infective agents
For many years, alcohols and aldehydes have been utilized as antiseptics and disinfectants.
- Isopropyl alcohol is mainly used to disinfect the skin and surgical tools in hospitals.
- Formaldehyde solution(Formalin) also shows germicidal action. Its mechanism of action is believed to involve the alkylation of the nucleophilic functional groups of proteins and nucleic acid.
- Glutaraldehyde solution is a commonly used chemical for the sterilization of equipment and instruments that can not be autoclaved.
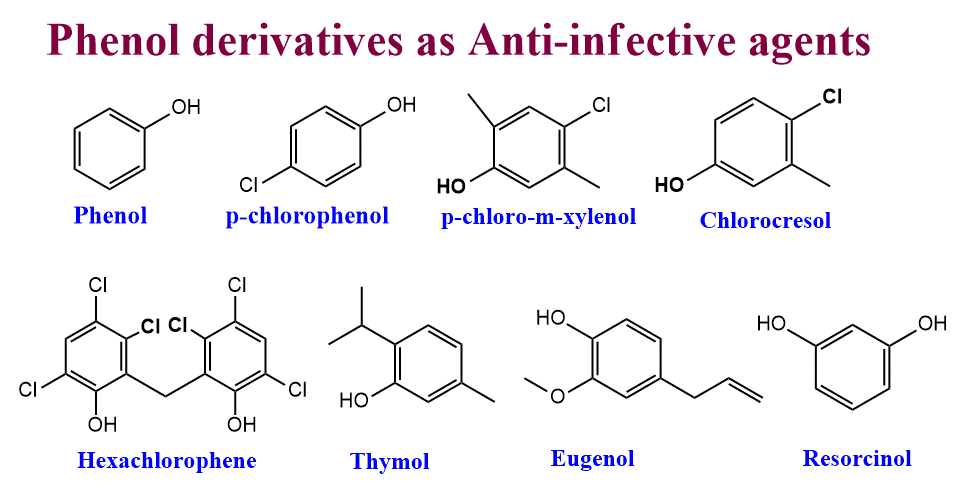
Phenol derivatives as Anti-infective agents
Not only phenol but also its derivatives show considerable germicidal and bactericidal activity. Therefore, it is still used as a USP standard(US Pharmacopeia) reference to compare the germicidal activity of other substances.
- Phenol
- Liquified Phenol
- p-chlorophenol
- p-chloro-m-xylenol
- Hexachlorophene
- Cresol
- Chlorocresol
- Thymol
- Eugenol
- Resorcinol
- Hexylresorcinol
Mode of action
Phenol and its derivatives act on bacteria or germs and these chemicals denature proteins of bacteria and may break the cell membrane of bacteria and kill them.
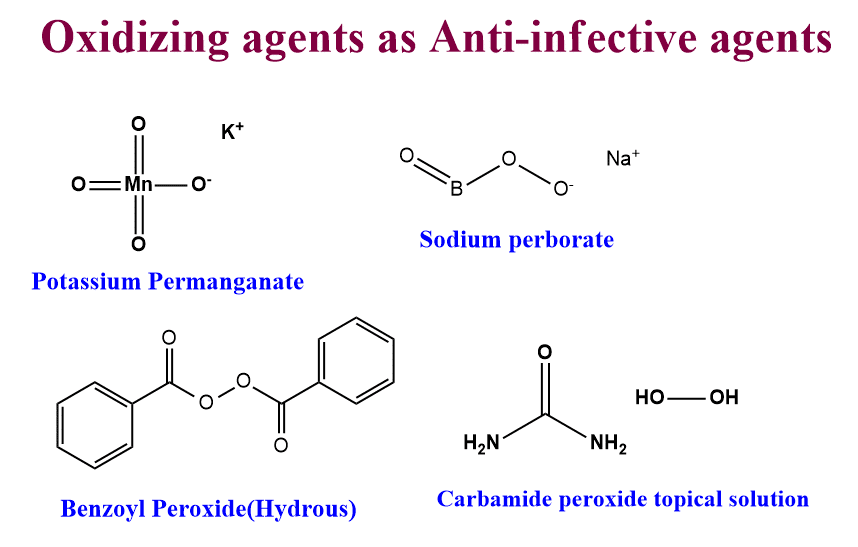
Oxidizing agents as anti-disinfectant agents
Some oxidizing agents can be used as germicidal agents and their potency depends on their ability to liberate oxygen in the tissue.
- KMnO4
- H2O2
- Sodium perborate
- Carbamide peroxide topical solution
- Hydrous Benzyl peroxide
Mode of action
Oxidizing agents react in the tissue to generate oxygen and oxygen free radicals. The formation of an oxygen bubble in reaction removes debris from the wounds. Moreover, some oxidizing agents directly react with the proteins of microorganisms and denature them.
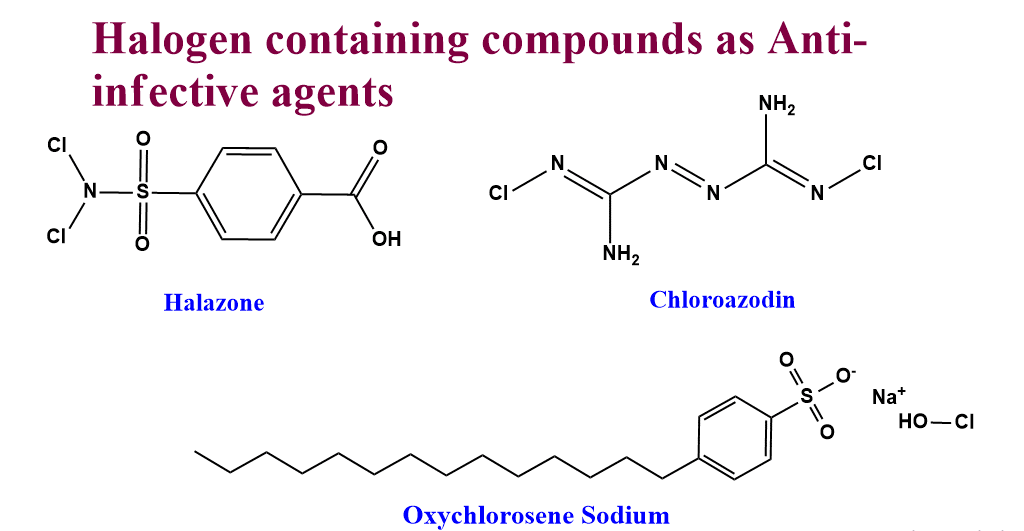
Halogen-containing compounds as anti-infective agents
There are some specific halogen-containing compounds that show antiseptic properties.
- Iodophors: The complex formed by mixing iodine and some nonionic and cationic surfactants is called iodophor. These are active bactericidal and fungicidal.
- Povidone-iodine: It is a charge transfer complex of iodine with nonionic surfactant polyvinylpyrrolidone(PVP).
- Halazone
- Chloroazodin
- Oxychlorosene sodium
Mode of action
The compounds probably act to inactivate the proteins of microorganisms by the iodination of the aromatic residue and oxidation.
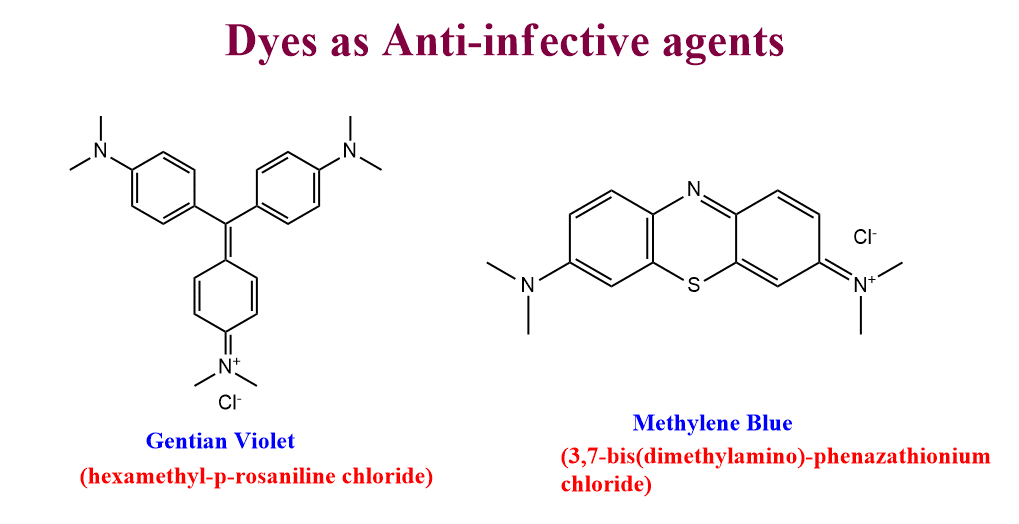
Dyes as anti-infective agents
Before the discovery of sulfonamides and antibiotics, organic dyes were used very extensively as anti-infective agents. The most common of them are listed below.
- Gentian violet
- Methylene Blue
- Basic Fuchsin
Mercury compounds as anti-infective agents
The compounds of mercury and its derivatives have been used in medicine for many years. There are two general classes of mercurials. The first class includes the compounds with at least one carbon-mercury bond and these don’t ionize readily. The second class includes the compounds with mercury bonded to heteroatoms and these ionize partially and completely.
- Inorganic salts of mercury: HgCl2(Mercuric chloride), Hg2Cl2( Mercurous chloride)
- Ammoniated mercury[Hg(NH2)Cl]
- Nitromersol
- Thimerosal
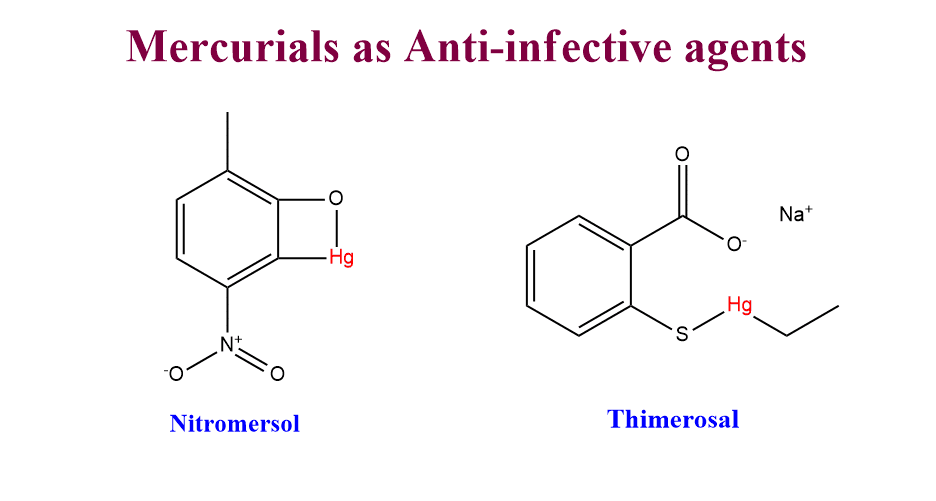
Mode of action
These compounds react with sulfhydryl(-SH) groups in enzymes and other proteins to form covalent compounds.
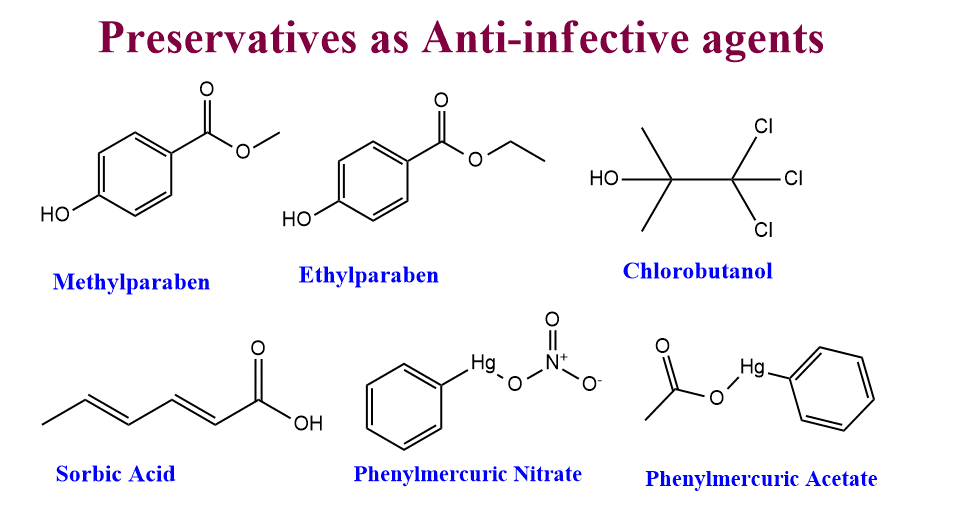
Preservatives as anti-infective agents
To avoid microbiological contamination, preservatives are added to a variety of dosage forms and cosmetic preparations. An ideal preservative would be non-toxic, compatible with other preparation ingredients, and efficient at low doses against all potential pathogens.
- p-hydroxybenzoic acid derivatives(Methylparaben, Ethylparaben)
- Chlorobutanol
- Sorbic acid
- Phenylmercuric nitrate
- Phenylmercuric acetate
References
- Brik, H., et al.: Nystatin. In Florey, K. (ed.). Analytical Profiles of Drug Substances, vol. 10. New York, Academic Press, 1981, p. 513.
- Oxford, A. E., et al.: Biochem. J. 33:240, 1939.
- Jawetz, E.: Principles of antimicrobial drug action. In Katzung, B. G. (ed.). Basic and Clinical Pharmacology, 6th ed. Norwalk, CT, Appleton & Lange, 1995.
- Jernigan, J. A., and Pearson, R. D.: Antiparasitic agents. In Mandell GL, Bennett J. E., and Dolin R. (eds.). Principles and Practice of Infectious Diseases, vol. 1, 4th ed. New York, Churchill-Livingstone, 1995.
