Table of Contents
ToggleX-ray Diffraction (XRD) is a non-destructive analytical technique used to extensively characterize the crystal structure of solids, defects, and stresses. It provides chemical information for both phase analysis and elemental analysis and helps to find the geometry of molecules (nanomaterials) using X-rays. It also determines the orientation of a single crystal or grain, measures the size, shape, and internal stress of small crystalline regions, and measures the average spacing between layers or rows of atoms.
An incident beam of X-rays interacts with one another as it exits a crystal due to its atomic planes. The phenomenon is known as X-ray diffraction. W.L Bragg and his father W.H. Bragg explained this phenomenon to calculate interatomic distances from X-ray diffraction patterns. According to Bragg’s equation, “When an x-ray strikes a crystal surface, its angle of incidence, θ, reflects back with the same angle of scattering, θ, and leads to constructive interference when the path difference, d, is equal to a whole number, n, of wavelengths”.
nλ = 2d sinθ
where d is the interplanar distance and λ is the wavelength of the X-rays.
Moreover, the size of the particle or average grain is estimated using XRD by using Scheerer’s formula:
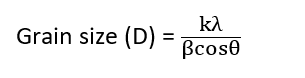
where, k = Scheerer’s constant whose value for spherical crystal is around 0.9, λ = wavelength of X-ray used, θ = diffraction angle, = full width at half maximum intensity (in radian) of the intensity profile.
It is also important to note that X-ray diffraction provides only an average crystallite size.
What is X-ray Diffraction?
When X-rays are passed through a narrow slit or obstructed by a small obstacle, they spread out or bend around the edges. This phenomenon of X-rays is called diffraction. If the set of evenly placed slits or periodic structures is used, the scattered X-rays interact with each other resulting in the formation of a series of bright and dark bands or fingers or patterns. The dark band results due to destructive interference and the bright bands due to constructive interference between waves. The dark band results, in fact, due to two waves being out of phases that their amplitude cancels partially or fully depending on the phase difference.
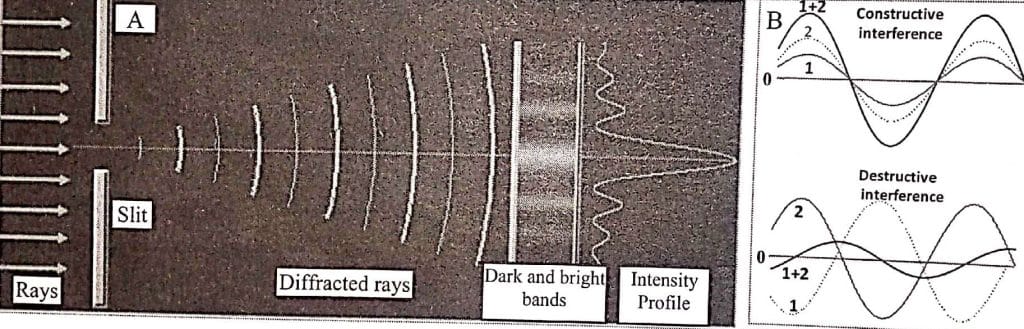
Experimentally, it is found that diffraction becomes more efficient when the wavelength of x-rays (in the range of 0.01-10 nm) is equal or comparable to the width of the slit. This phenomenon of X-ray diffraction is used for the characterization of internal structures or arrangements of materials. Light scattering, also known as dispersion of light, occurs when a material either lacks periodic features or has periodic structures larger than the X-ray wavelength. In nanoresearch, the following X-ray diffraction (XRD) and scattering methods are employed:
- Single crystal X-ray diffraction
- Powder X-ray diffraction
- Small angle X-ray scattering
Single Crystal XRD
Atoms are arranged in periodic arrays (or planes) in crystals, and their spacing is typically between 0.02-2 nm, or about the same as the wavelength of an X-ray. The periodic arrays of crystal diffract arrays cause the formation of bright and dark band patterns known as X-ray diffraction patterns, just as the diffraction of X-rays by a set of evenly spaced slits. The diffraction pattern gives information regarding the internal arrangement of atoms in crystalline materials. This is the fundamental of X-ray crystallography.
Single Crystal XRD Principle
The electromagnetic field of X-rays causes the electrons in an atom to oscillate when they are incident on a crystal. As a result, X-ray oscillation is elastically scattered. A heavy atom that contains a large number of electrons scatters X-rays more than a light atom.
Let us consider two parallel X-rays 1 and 2 are made incident on two different planes of crystal at an incident angle (θ).
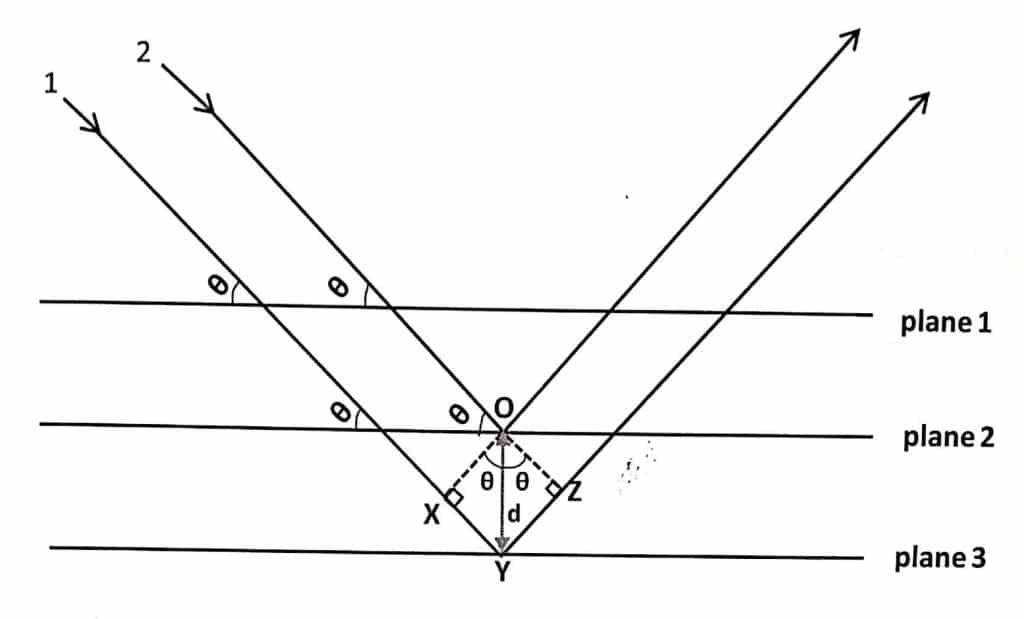
The total path difference between two x-rays that escape from the crystals (assuming the law of reflection) is given by:
Path difference = XY + YZ
From triangle OXY, XY = dsinθ
From triangle OYZ, YZ = dsinθ
where, d = OY is inter-planar spacing (distance of separation between two planes. Thus,
Path difference = dsinθ + dsinθ = 2dsinθ
A constructive interference that results in the bright band (also called Bragg’s peak), is observed when the path difference between diffracted X-rays is equal to:
nλ = 2d sinθ
where, λ = wavelength of X-ray and n = 1, 2, 3…………..called the order of reflection.
Single Crystal XRD Instrumentation
In an experiment, a monochromatic beam of x-rays is focused on a single crystal that is mounted on a goniometer. The goniometer allows the crystal to be rotated at a precise angle. Using an X-ray-sensitive camera or detector, the angle of the diffraction patterns is calculated for each revolution. The two-dimensional diffraction pattern or images taken at all possible angles are converted to three-dimensional electron density maps with the help of Fourier transformation.
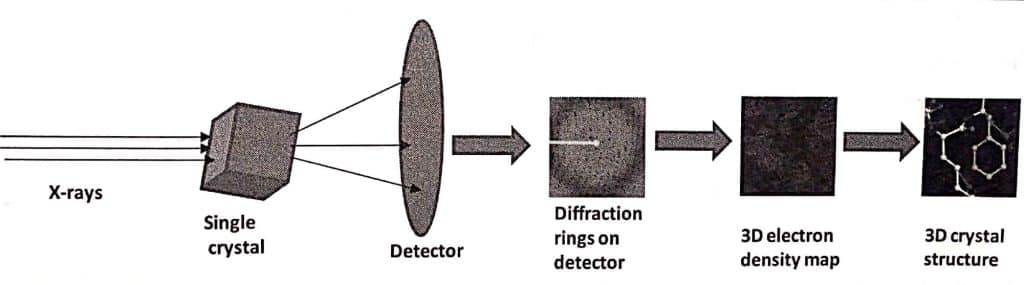
The electron density map indicates the potential elemental composition of a crystal. If the crystal’s quality is good, it will effectively diffract X-rays. In such cases, the position of atoms in the crystal can be determined with high precision resulting in a high-resolution crystal structure.
This method can be used to study any material that can be crystallized into a single high-quality structure. It is extensively used to determine the structure of complex biomolecules. Moreover, single-crystal XRD is the source of almost all crystal structures of biomolecules that are known to us.
Application of single crystal XRD
- Determination of the position of atoms present in a crystal.
- Crystallographic information such as unit cell dimensions, inter-planar spacing, symmetry, etc. can be obtained by using single crystal XRD.
- For knowing the nature of bond and bond angle
Powder XRD
If nanomaterials to be characterized do not form a good quality single crystal but exist as powder, then it can be characterized using powder XRD.
Powder XRD Principle
The basic principle of powder XRD and single crystal XRD are the same. In a brief, the basis of powder XRD is the elastic scattering of X-rays by periodic structures present in a crystal. When Bragg’s law is obeyed, the scattering increases to its maximum.
Data are plotted as scattering intensity (I) versus scattering angle which is set as twice the incident angle, with 2θ in the range of 10-70. Depending on the nature of the sample series of peaks (collectively called patterns) are obtained at specific 2θ values called diffractograms. Each peak in the diffractogram corresponds to a scattering from a specific plane hkl. Information such as inter-planar spacing, unit cell dimensions, average grain, and strain size can be obtained if the diffractogram is analyzed.
Powder XRD Instrumentation
Similar to single crystal XRD, an X-ray monochromator beam is focused on a crystal powder sample that is placed on a goniometer. The sample can be rotated at a precise angle by using a goniometer. Using an X-ray-sensitive camera or detector, the diffraction intensity at 2θ is determined for each rotation angle.
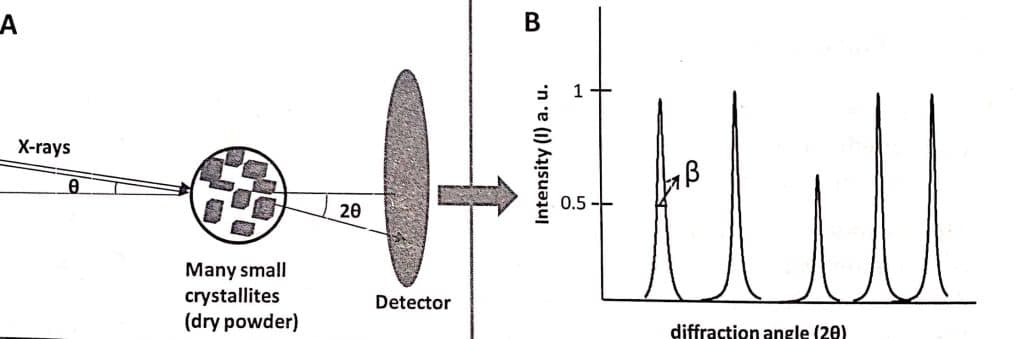
This method can be used to examine any sample that is present in dry powder form. Similar to single crystal XRD, powder XRD is a non-destructive technique.
Application of powder XRD
- Lattice parameters such as unit cell dimensions and inter-planar spacing can be analyzed using powder XRD.
- Average grain or particle size can be estimated using Scherrer’s formula.
- It helps to know whether the strain is present in the sample or not.
- Study of change in crystalline phase via the position of the diffraction peak
Single crystal XRD Vs Powder XRD
The difference between single crystal XRD and powder XRD are:
| Single crystal XRD | Powder XRD |
| The sample should be in crystalline form. | The sample should be in dry powder form. |
| The material should be crystallized into a single high-quality structure | Easier and more convenient than single-crystal XRD. |
| Used to obtain crystallographic information such as unit cell dimensions, inter-planar spacing, symmetry, etc. | Used to know lattice parameters and average grain or particle size. |
X-ray Diffraction Video
References
- M. W. Woolfson, Ed, An introduction to X-ray crystallography, Cambridge university press, Cambridge, UK (1997).
- C. F. Holder and R. E. Schaak, Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials, ACS Nano (2019)