Table of Contents
ToggleAn operation that changes a system from one state to another state is called a thermodynamic process. A system can be changed from its one equilibrium state to another equilibrium state in different ways. The different processes frequently encountered in thermodynamics are as follows:
- Isothermal process
- Isobaric process
- Isochoric process
- Adiabatic process
- Cyclic process
These different processes are described in short one by one as follows:
Isothermal process
A process is said to be isothermal if the temperature of the system remains constant during each step of the process.
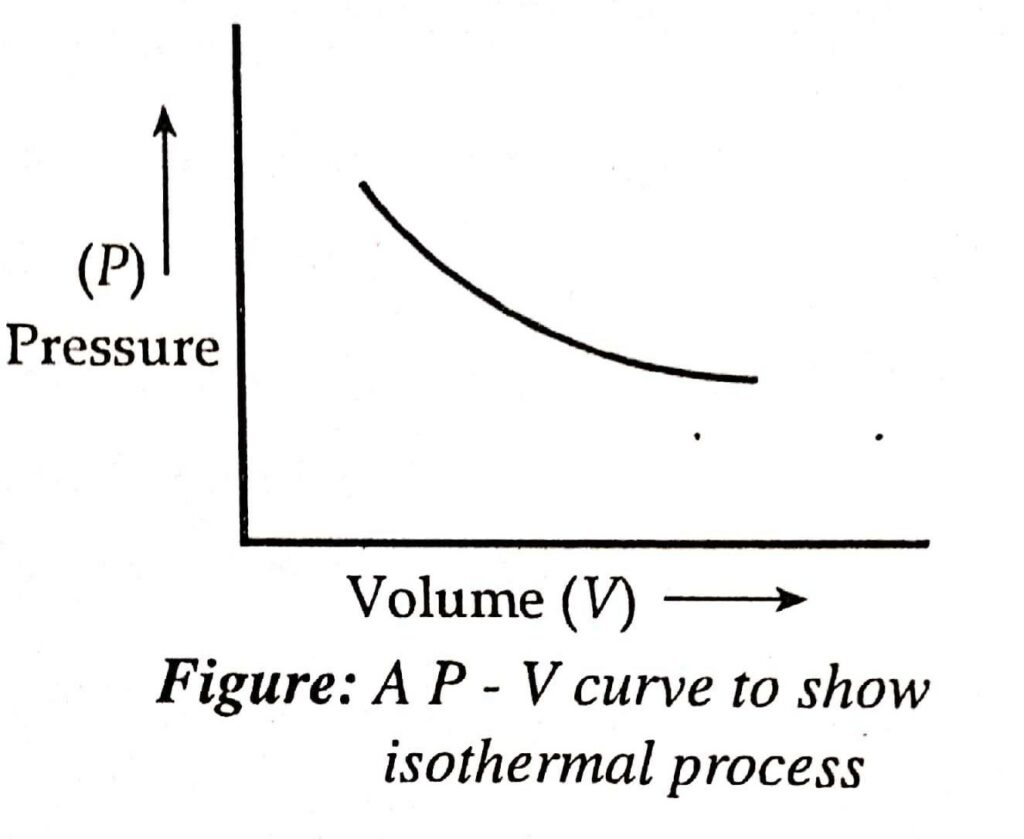
- For an isothermal process, dT = 0
- The isothermal process may be carried out by placing the system in a thermostat.
- Heat can flow from the system to the surrounding or vice-versa to keep the temperature of the system constant. When a gas is compressed suddenly then heat is produced. If the temperature is to be kept constant and the compression is carried out slowly, then the heat produced should be removed.
- When gas is allowed to expand slowly then work is done by the system and heat is absorbed. If the temperature is to be kept constant then heat should be supplied from outside.
- For ideal gas at the isothermal condition, Boyle’s law is given by the relation, P1V1 = P2V2 or PV = constant.
Isobaric process
A process is said to be adiabatic if no heat enters or leaves the system during any step of the process.
- For isobaric process, dp = 0
- This process is carried out in a vessel fitted with a weightless and frictionless piston.
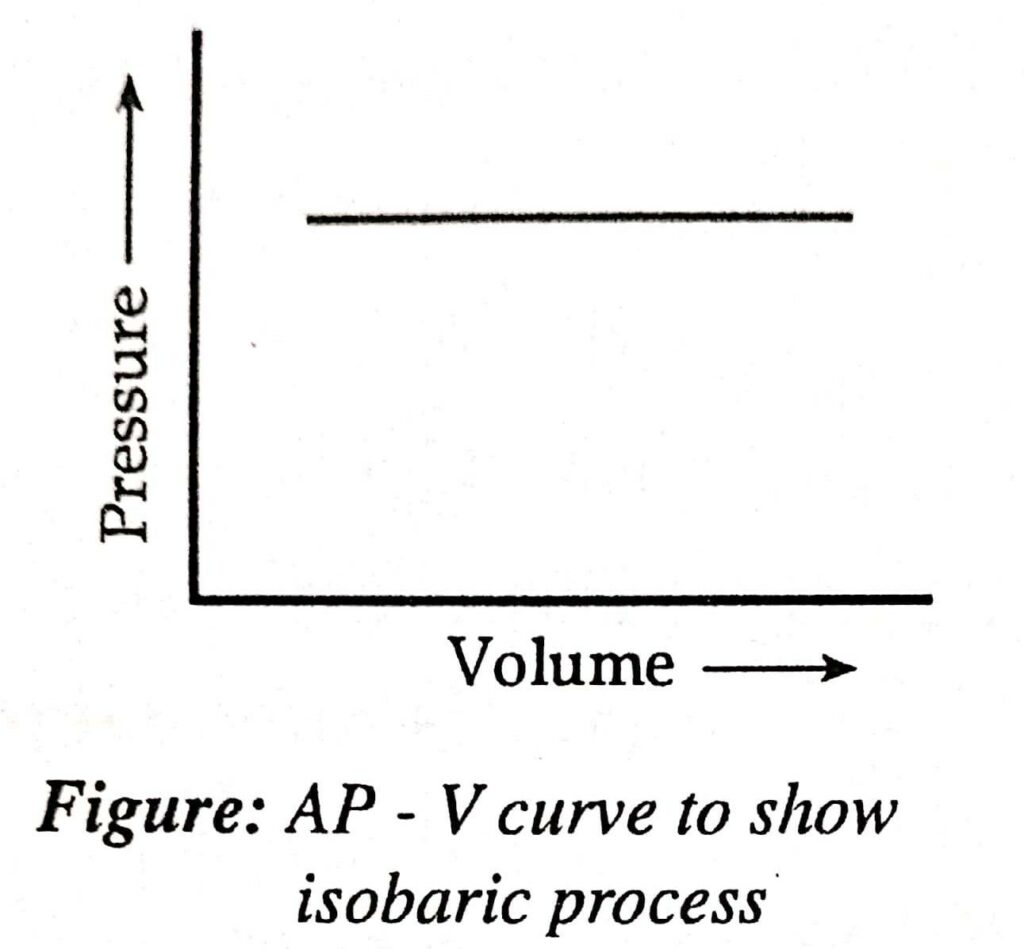
It is possible for the system volume to alter during the isobaric process. Let’s take a 1:1 mixture of hydrogen and chlorine in a cylinder that is fitted with a weightless, frictionless, and airtight position as an example. The reaction is carried out by the passage of light.
H2(g) + Cl2(g) → 2HCl(g)
During this chemical reaction, there is no change in the volume of the system and the piston remains stationary to keep the pressure constant.
In the next experiment, let us take a mixture of H2 and O2 in a 2:1 ratio by volume in a cylinder fitted with a weightless, frictionless, and airtight position. When an electric spark is passed then a chemical reaction takes place.
2H2(g) +O2(g) → 2H2O(g)
The chemical reaction takes place through a decrease in the volume of the system. To keep the pressure constant, the piston should move down.
In another example of N2O4(g) → 2NO2(g), the reaction takes place with an increase in volume. So to keep the pressure constant, the piston should move up.
Most of the experiments conducted in the lab are carried out at constant pressure.
Isochoric Process
A thermodynamic process is said to be isochoric when the volume of the system is kept constant during each step of the process.
- For isochoric process, dV = 0
- The isochoric process is carried out in a cylinder fitted with a fixed piston.
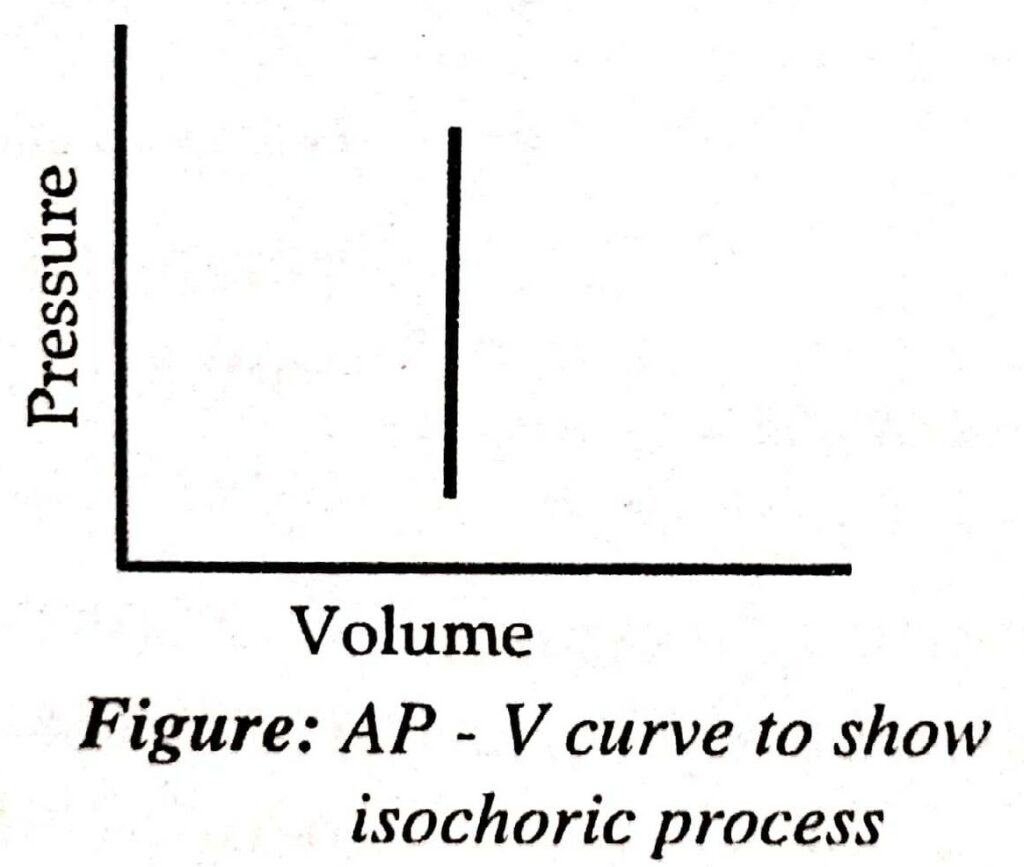
In the isochoric process, the pressure of a system may change. Let us take an example of where dissociation of PCl5 takes place.
PCl5(g) → PCl3(g) + Cl2(g)
For the dissociation of each molecule of PCl5, two moles of products are formed and volume increases. To keep the volume constant, the piston should be fixed. In this case, the pressure of the system increases to keep the volume fixed.
Adiabatic Process
A thermodynamic process is said to be adiabatic when there is no exchange of heat between the system and the surrounding.
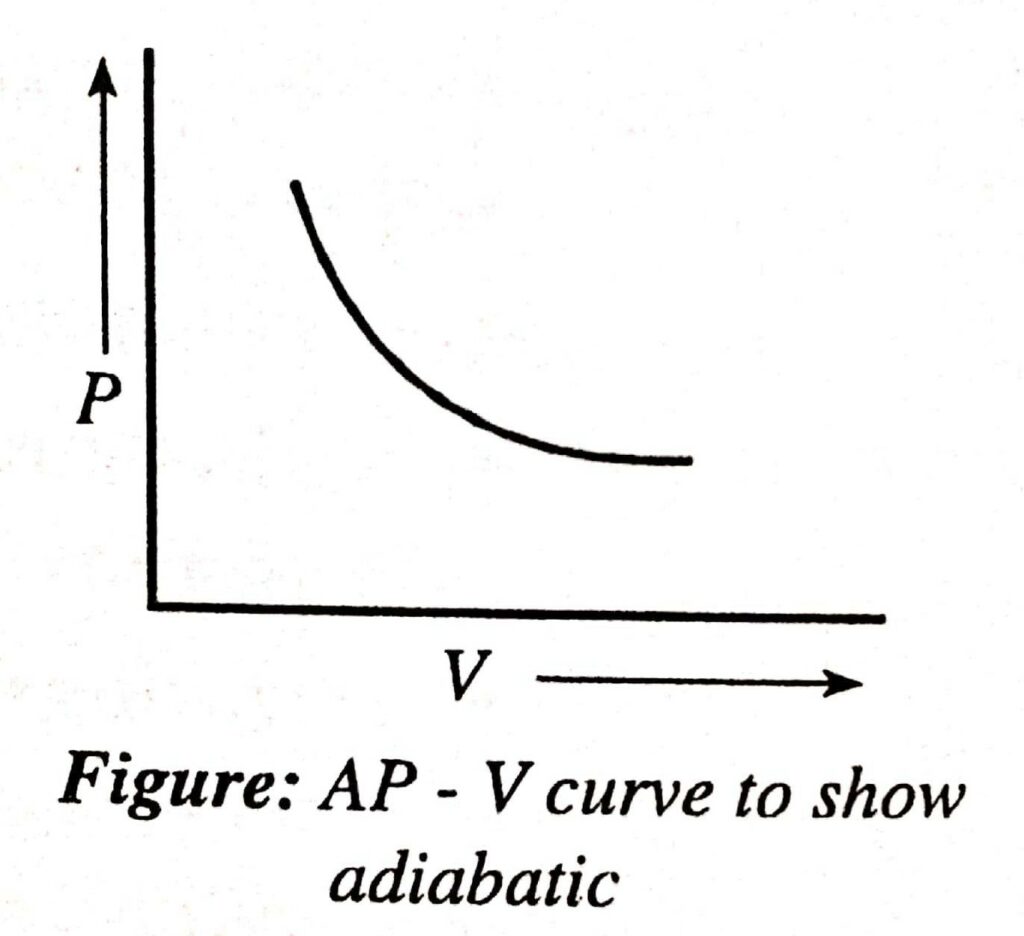
- The adiabatic process is carried out in a cylinder enclosed by adiabatic walls so that heat is not exchanged.
- During adiabatic process, the temperature of a system may change.
- If the process is exothermic, the heat evolved remains in the system by the increase in temperature. If the process is endothermic, the heat absorbed is supplied by the system itself by the decrease in temperature.
- For adiabatic process, PV = constant i.e. P1V1γ = P2V2γ
- The curve of the adiabatic process is steeper than that of the isothermal process.
Cyclic process
When a thermodynamic process is carried out in such a manner that after a series of state of changes the system is brought to its original state, then the process is known as a cyclic process.
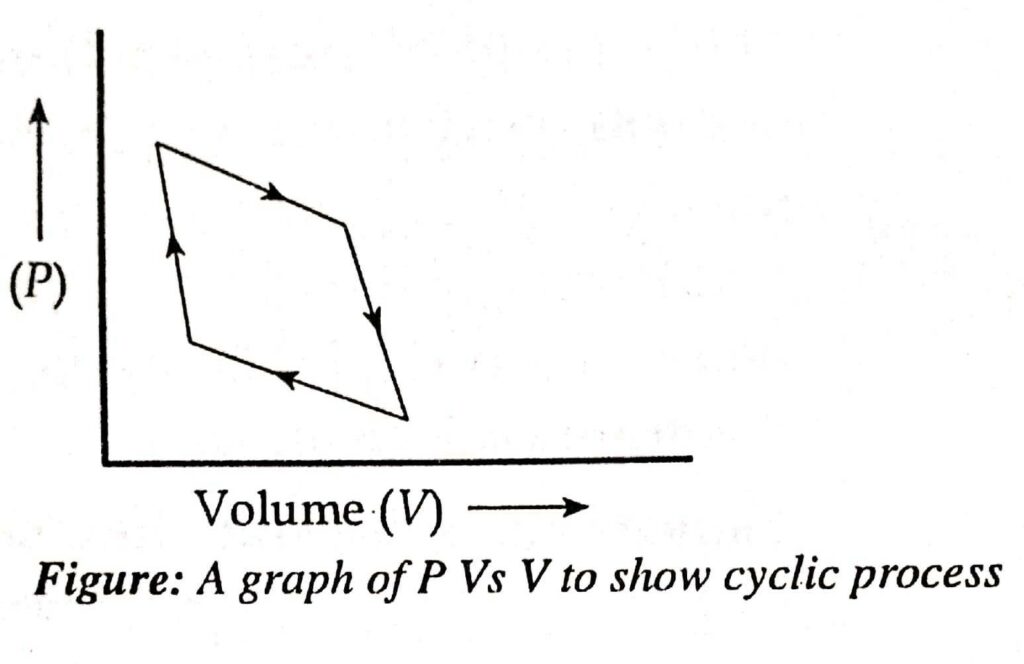
- Net change of internal energy (dE) = 0
- Net change of internal enthalpy (dH) = 0
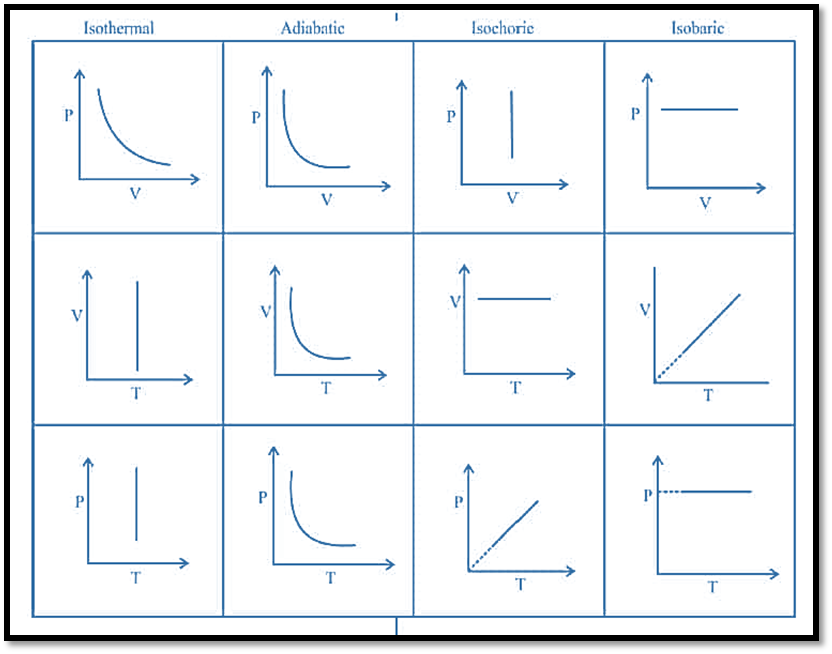
Besides these, all thermodynamic processes can be represented as:
Reversible and Irreversible Processes
A process carried out infinitesimally slowly so that the driving force is only infinitesimally greater than the opposing force is called the reversible process. During a reversible process, there is no increase in the system’s entropy, and the overall system is in perfect equilibrium with its surroundings. The system’s operation is reversible if,
W = ∫PdV, where P is the pressure and V is the volume
A reversible process may be regarded to proceed from the initial state to the final state through an infinite number of infinitesimally small intermediate stages and the system remains in an equilibrium state at the initial, final, and intermediate stages. A reversible process cannot be realized in the process because it would require infinite time for its completion. Hence almost all processes occurring in nature or laboratory are irreversible. A reversible process, therefore, remains imaginary and theoretical.
Any process which does not take place infinitesimally slowly is said to be an irreversible process. It is impossible for a system to revert to its initial condition if the entropy of the system increases as a result of a thermodynamic process. Thermodynamic alterations will also affect the surrounding area, rendering it permanently altered from its original state. Thus, the system’s entropy will increase during this process.
Thermodynamic Process Video
References
- Atkins, P.W. and Julio de Paulo, Atkins’ Physical Chemistry, Oxford University Press, UK, Indian Edition 9, 2011.
- R. Chang, “Physical Chemistry for the Chemical and Biological Sciences”, University Science Books, Sausalito, California (2000).






