Table of Contents
ToggleWhat is Sublimation?
Sublimation is the process of converting substances from a solid to a gaseous state without passing through a liquid state. It Is the most delicate method for the separation and purification of solid organic compounds.
Sublimation examples
- Sublimation is a term used in the water cycle to describe the process of snow and ice converting to water vapor in the air without first melting into water.
- At 100 degrees Celsius, iodine sublimates from a solid to a toxic purple gas.
Sublimation process
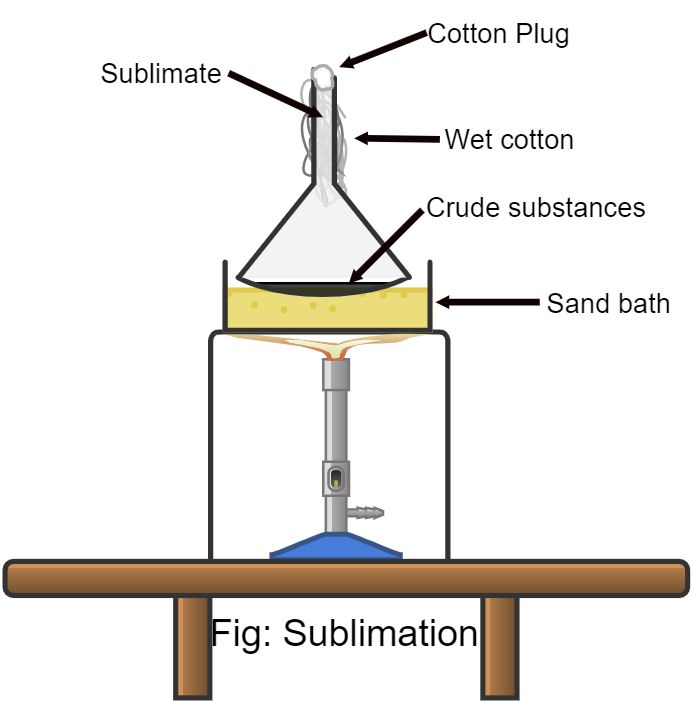
For separation and purification of substances, which on heating pass directly into vapor state without melting and on cooling solidify, the technique of sublimation is used. Sublimation occurs when the atmospheric pressure exerted on the substance is too low to prevent the molecules from escaping from the solid-state. The substance is taken in a watch glass over which is kept in an inverted funnel with the cooling arrangement. The watch glass is then heated over a sand bath. The substance which sublime deposits as pure solid on the inner surface of the inverted funnel.
Sublimation diagram
The arrangement used for the sublimation experiment is shown in fig.
FAQs
What is the process of sublimation?
Solid substances which on heating pass directly into vapor state without melting and on cooling solidify, the technique of sublimation is used.
What is the sublimation with example?
Sublimation is the process of converting substances from a solid to a gaseous state without passing through a liquid state. e.g. ice directly convert into vapor without melting.