Table of Contents
ToggleProtein purification by ion-exchange chromatography
Ion-exchange chromatography is a type of chromatography that is widely used in the purification of the protein, amino acids, etc. Ion exchange chromatography separates biomolecules according to the types and strength of their charge. There are two types of ion exchange viz. cation exchange and anion etc.
(1) Principle of protein purification
Protein purification by ion-exchange chromatography is based on the following principle:
Most of the proteins have net charges that depend on the amino acid compositions and pH of the buffer used. At the isoelectric point, proteins have net-zero charges. But, at pH greater than the isoelectric point of a given protein, the protein becomes negatively charged. Similarly, at pH less than the isoelectric point, the protein becomes protonated and positively charged. Such charged molecules bind to oppositely charged resins(stationary phase). This interaction is used for the separation and purification of various proteins.
Some proteins weakly bind and some bind strongly depending on their affinity of exchange or interaction. When protein molecules bind to resins, by using a salt gradient, the bonded proteins are eluted from the resin. The salt ions compete with protein for binding sites. Therefore, the proteins with weak ionic interactions are the first to elute from the column. For example, in the case of cation exchange chromatography, proteins that are less positively charged start to elute first. With an increase of the concentration to salt the proteins with stronger ionic interaction elute later from the column.
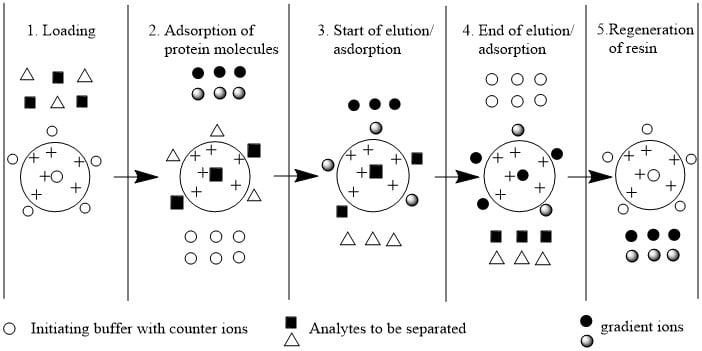
(2) Steps (procedure) of separation and purification of proteins
Step 1: Equilibrium of stationary phase (resin)
First of all, the resins are maintained at equilibrium by using suitable pH and salt buffer so that the proteins/amino acids from the mixture can interact and bind with the counterion of resins.
Step 2: Application of sample
The mixture of proteins/amino acids is applied in a column containing an equilibrated stationary phase so that proteins are bound to the resin and unbound proteins wash out using a suitable solvent.
Step 3: Elution
When proteins(amino acids) are bound to the resins, the bound proteins to resin are now separated from them using suitable eluent. The proteins carrying the lowest net charge at selected pH will be eluted first and which is collected from the column. On increasing the salt concentration of the buffer, the proteins with the highest charge will be eluted.
- Elution with salt gradient: Addition of salts generation lots of ions which compete which proteins for the functional groups on the stationary phase.
- Elution with pH change: By changing pH of in the column, the net charge of the adsorbed proteins decreases and decreases their attraction to the stationary phase and enhance the elution.
Step 4: Regeneration of Resin/ Stationary phase
After the elution of all bound protein molecules, the resin is made again active by washing with suitable buffer at suitable pH so that we can use these resins again for separation.