Table of Contents
ToggleClassical Carbocations
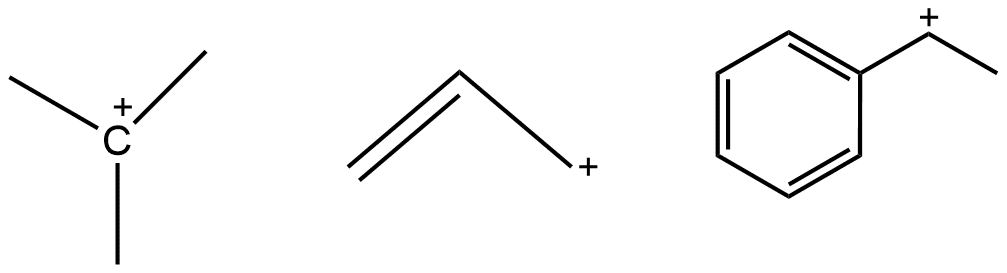
Positively charged carbon with three bonds stabilized by the +I-effect, hyperconjugation, resonance, and the presence of a double or triple bond in the allylic or benzylic position.
Example:
Non-classical carbocations
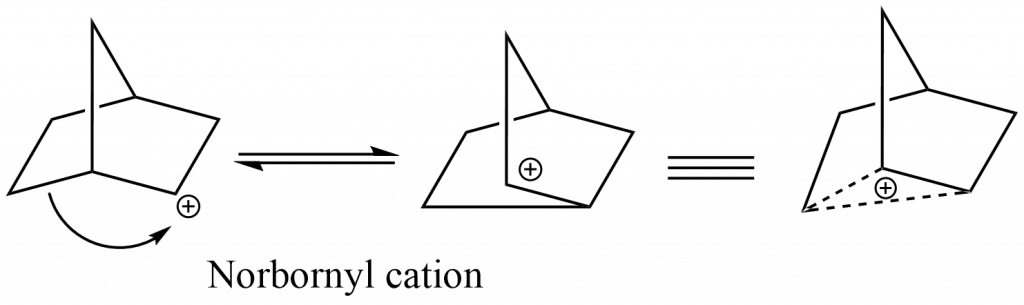
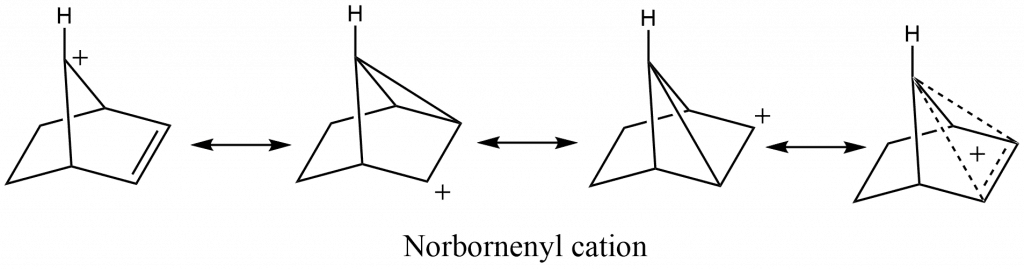
Non-classical carbocation or bridged carbocation is a carbocation that is stabilized by the neighboring group participation of C=C, C-C, and C-H-bonds, and the positive charge is delocalized by double or triple bonds that are not in the allylic position or by a single bond.
Examples:
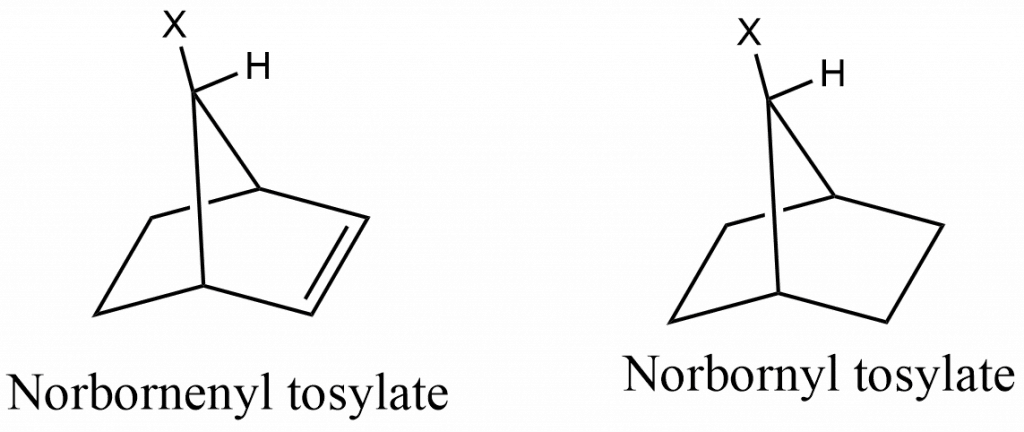
The acetolysis of norbornenyl tosylate, which is 1011 times faster than norbornyl tosylate, is the most compelling evidence that the C=C double bond acts as a neighboring group.
The Neighboring Group Mechanism
When using specific substrates, it is often found that the rate of reaction is higher than predicted, and the configuration at a chiral carbon is retained rather than inverted or racemized. In these situations, the leaving group usually has a group with an unshared pair of electrons. In these situations, the leaving group usually has a group with an unshared pair of electrons. The mechanism operating in such cases is called the neighboring-group mechanism and consists essentially of two SN2 substitutions, each causing an inversion so the net result is the retention of configuration.
The neighboring group functions as a nucleophile in the first step of this reaction, pushing out the leaving group but remaining attached to the molecule. In the second step, the external nucleophile uses a backside attack to displace the neighboring group:

Z, a neighboring group, is reported to be providing anchimeric support. The rate law followed in the neighboring-group mechanism is first-order, which means that Y isn’t involved in the rate-determining step. Because group Z is more available, Z attacks are faster than Y attacks. Y must collide with the substrate in order to respond, while Z is instantly available due to its location.
Example for Neighboring group mechanism
The hydrolysis of the 1,2-chlorohydrin indicated below with a base catalyst yields the 1,2-diol with configuration retention.
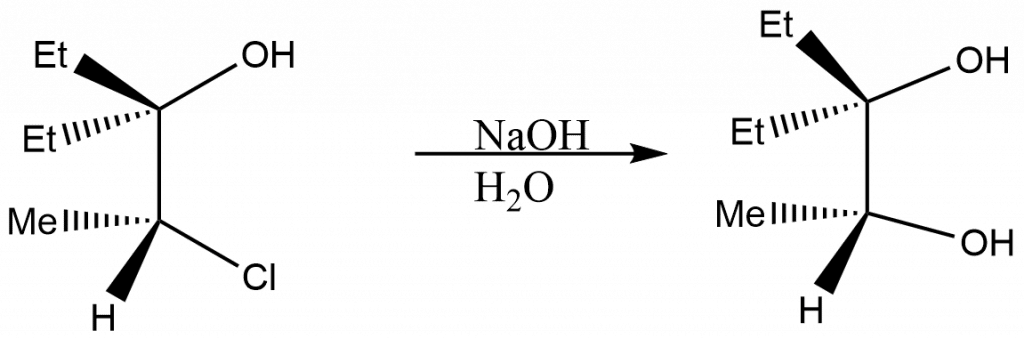
Mechanism

Sometimes rearrangement product can be obtained
References






