Table of Contents
ToggleNegishi coupling reaction is a palladium-catalyzed cross-coupling reaction that forms a carbon-carbon (C-C) bond by coupling organic halides or triflates with organozinc compounds. Professor Ei-ichi Negishi (1997) discovered this reaction and found it useful in the preparation of unsymmetrical biaryls with good yields.
The Negishi reaction, like the Heck reaction, can be used for the formation of carbon-carbon bonds during organic synthesis. Negishi cross coupling reaction involves the coupling of sp3, sp2, and sp carbons, making it somewhat distinct among Pd-catalyzed coupling reactions. Furthermore, this reaction can tolerate a variety of functional groups such as ether, olefin, ester, nitro, amines, nitriles, etc., thus possessing high functional group tolerance.
Negishi reaction is another versatile reaction that uses organometallics compounds of Zinc, Zirconium, and Aluminium in presence of a transition metal complex catalyst, mostly of palladium or nickel.
Negishi coupling reaction
Negishi coupling reaction is a cross-coupling reaction that involves an organozinc compound, an organic halide, and a nickel or palladium catalyst and creates a new carbon-carbon covalent bond. Not only organozinc compounds but also di-organozinc halides compounds can be used as starting material.
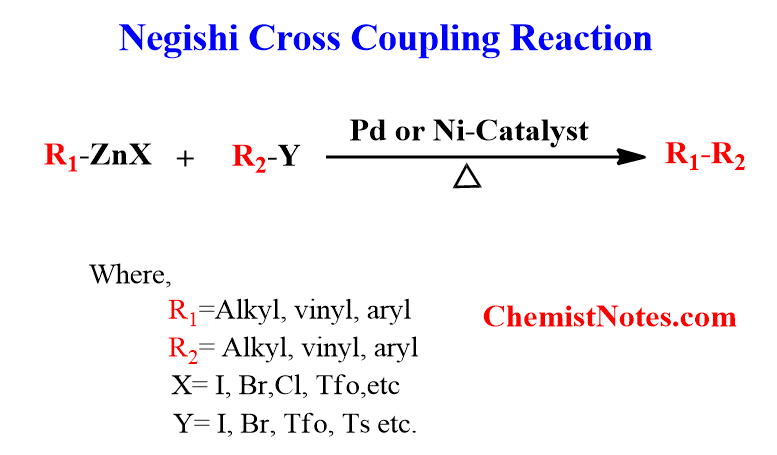
The metal catalyst for the reaction is usually palladium (0) species, though nickel is occasionally used. The ligand ‘L’ in the catalyst can be either triphenylphosphine or dppe or BINAP.
Note: Since organozinc compounds are moisture and air sensitive, the Negishi coupling must be performed in an inert atmosphere i.e. oxygen and water-free environment.
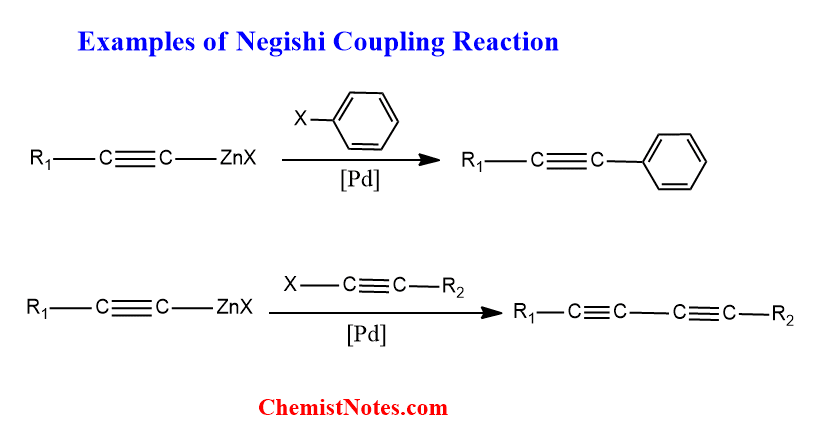
Negishi reaction examples
The Negishi reaction involves the joining of the coupling partners and some of such reactions are shown below:
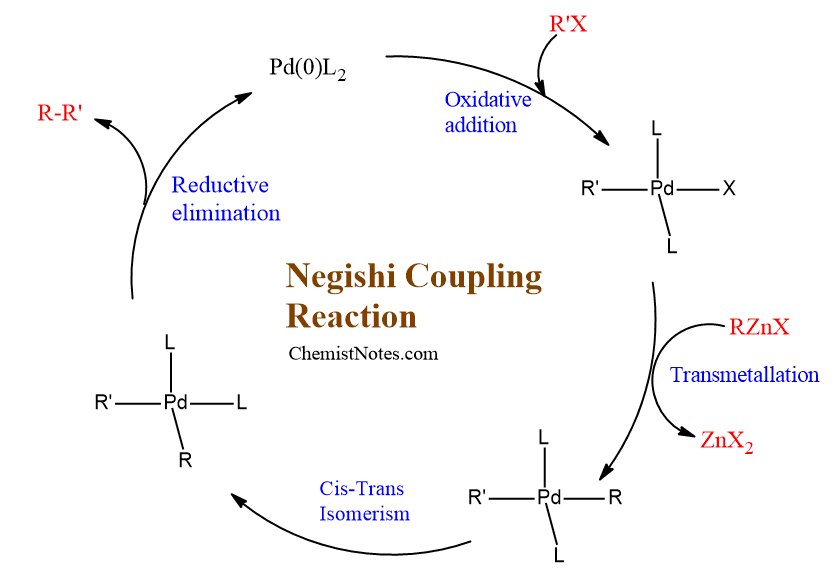
Negishi coupling mechanism
The commonly accepted mechanism of the Negishi coupling reaction is a catalytic cycle which consists of three major steps. The active catalyst in this process is Pd[0], and the reaction generally proceeds through (i) oxidative addition of the organic halide, (ii) transmetallation (an organometallic reaction that involves the transfer of ligand from one metal to another metal) with the zinc compound followed by (iii) reductive elimination leading to the formation of new C-C bonded compound and the Pd[0] catalyst.
Oxidative addition of organic halide to Pd-catalyst leads to the oxidation of Pd(0) species to Pd(II). The second step is transmetallation which involves the transfer of organic group from organic halide to the Pd-complex. The last step is reductive elimination which involves the formation of coupled product and regeneration of Pd-catalyst. The regenerated Pd-catalyst can enter into another catalytic cycle leading to the formation of C-C compound.
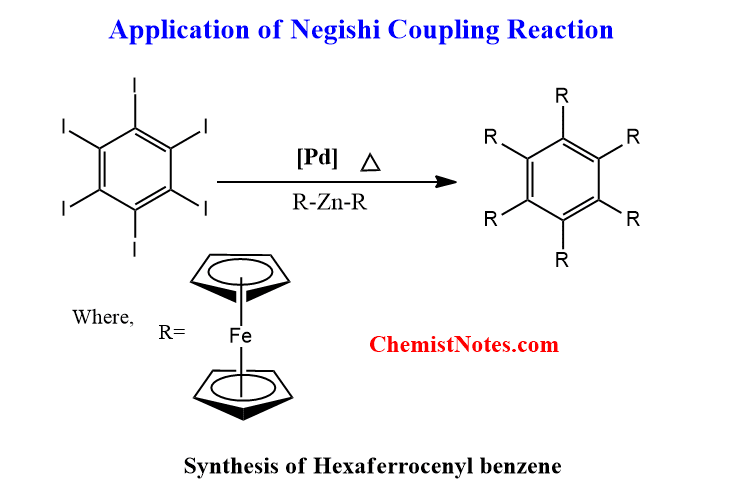
Application of Negishi coupling reaction
Negishi coupling reaction is not only restricted to the formation of biaryls rather it’s been widely used for the synthesis of natural and pharmaceutical products. It is due to the potentiality of this reaction for the formation of carbon-carbon bonds.
Some of the major applications of Negishi coupling reactions are:
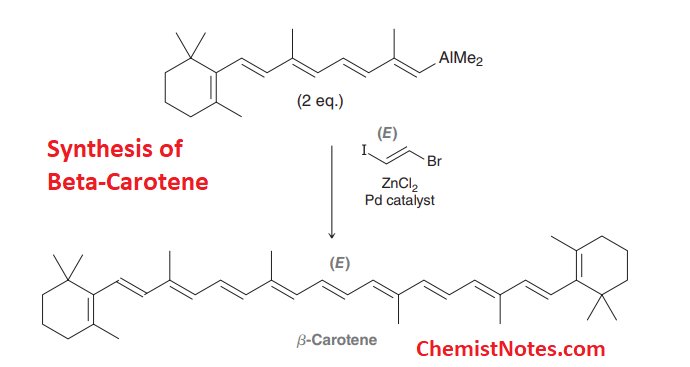
- Synthesis of Beta (β)-carotene.
2. Synthesis of Hexaferrocenyl benzene