Table of Contents
ToggleClaisen rearrangement, examples, mechanism, and its application have been discussed here. The claisen rearrangement was first reported by Luduig Claisen in 1912.
The claisen rearrangement
Claisen rearrangement is the sigmatropic rearrangement of allyl vinyl ether or allyl aryl ethers to form γ,δ– unsaturated carbonyl compound or O-allyl substituted phenols respectively.
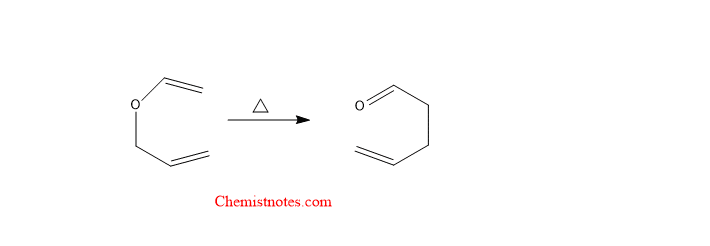
Characteristics of Claisen rearrangement:
- Mostly these reaction takes place via chair like transition state.
- These rearrangement occur at a relatively high temperature.
- The rate of reaction increases in presence of polar solvent.
- The subsituents on allyl vinyl ether determines the reactivity of corresponding allyl vinyl ether. If electron density group is present on the position of 1,2,4,6 then the rate of rearrangement increases. Similarly electron withdrawing group present on position 2,4,5 enhance the rate of the rearrangement.
- There are more than 20 types of modified claisen rearrangement. Some of them are discussed here.
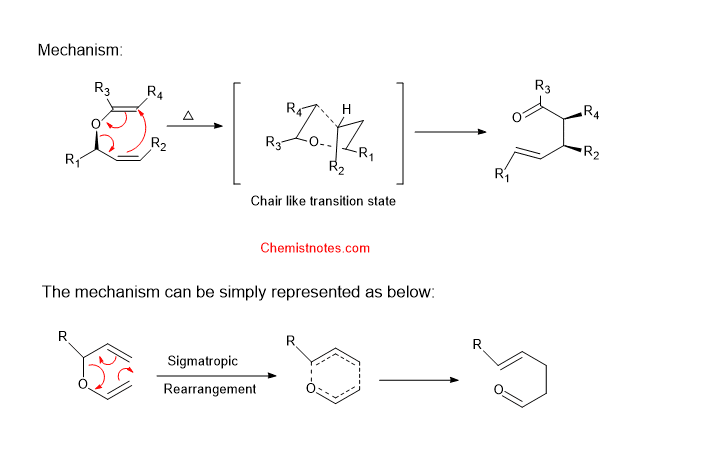
Claisen rearrangement mechanism
The mechanism of claisen rearrangement is concerted and takes place mostly via a chair-like transition state
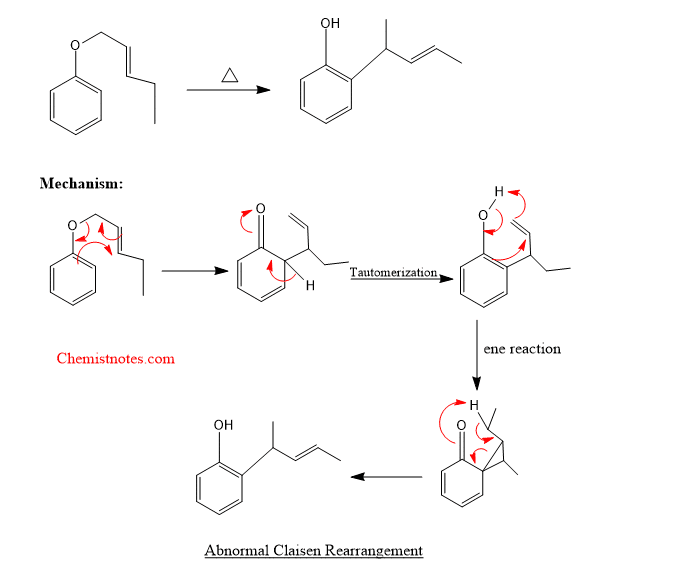
Abnormal claisen rearrangement
The product formed in normal claisen rearrangement, having β- carbon with it further undergoes rearrangement reaction to give a product in which the β-carbon is attached to the ring. Such a reaction is called abnormal claisen rearrangment. Let’s see an example of abnormal claisen rearrangement.
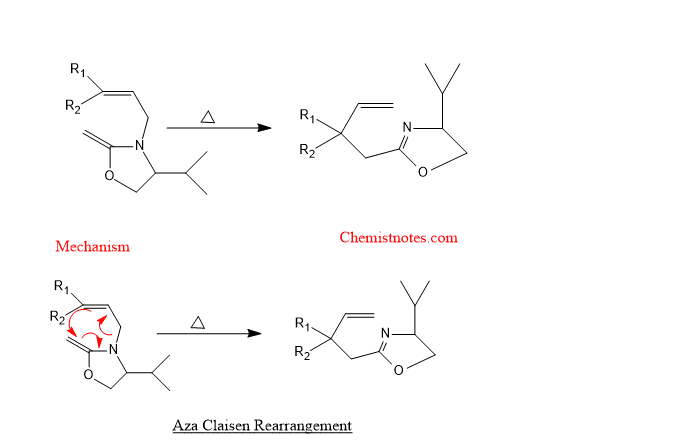
Aza claisen rearrangement
The reaction in which N-allyl enamines undergo [3,3]-sigmatropic rearrangement is called aza claisen rearrangement. Let’s see an example:
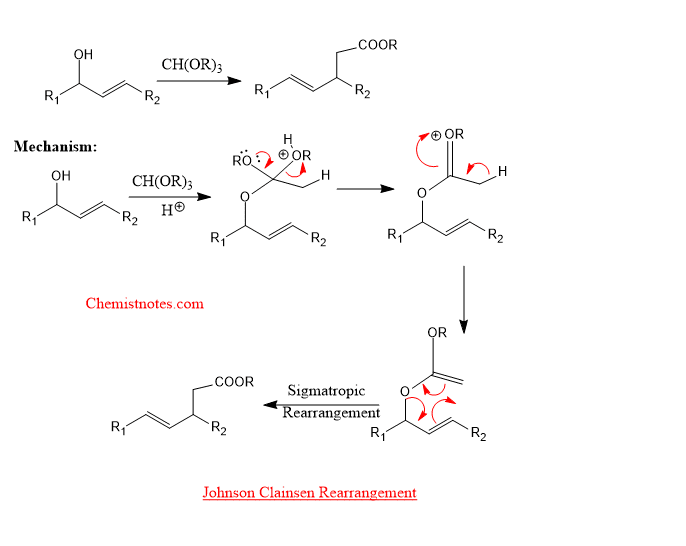
Johnson claisen rearrangement
When allylic alcohol is heated with an excess of trialkyl orthoacetate in presence of weak acid, then an orthoester is formed. The orthoester loses alcohol to give ketene acetal which on further rearrangement gives γ,δ- unsaturated ester. The example of johnson claisen rearrangement is given below:
Eschenmoser claisen rearrangement
When N,O-ketene acetals undergo [3,3]-sigmatropic rearrangement reaction then γ,δ- unsaturated amides is formed. Such reaction is known as eschenmoser claisen rearrangement. Let’s see one of the examples of eschenmoser claisen rearrangement.
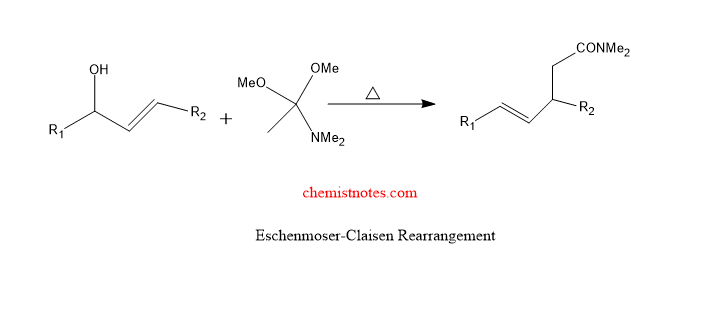
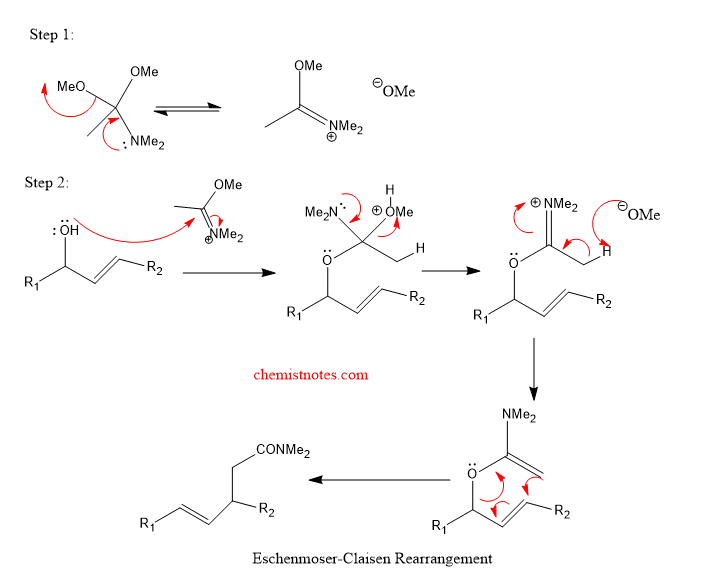
The mechanism of this reaction can be given as:
Ireland claisen rearrangement
When allylic ester enolate reacts with trimethylsilyl chloride then gives allyl trimethyl silyl ketene acetal which undergoes rearrangement reaction to give γ,δ- unsaturated carboxylic acid. Let’s see the following example.
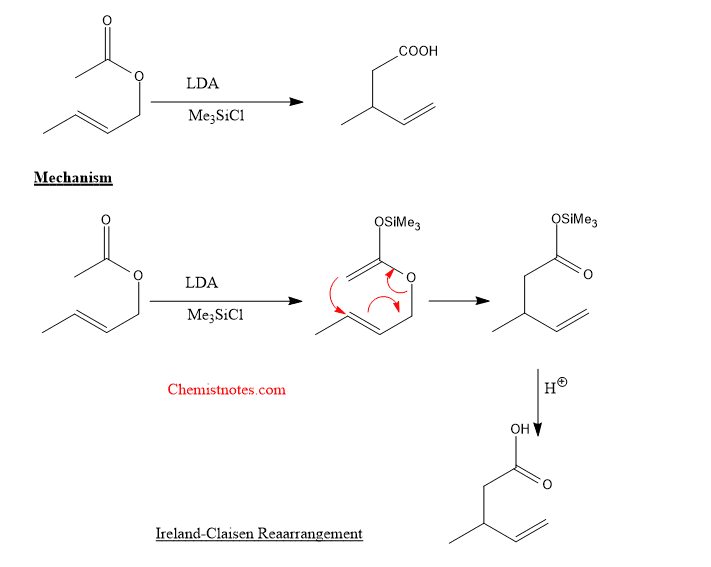
If you want to learn more about other rearrangement reactions such as Beckmann rearrangement, then click here.
Please comment if you have any problems related to this topic. Thank you.
Claisen rearrangement video
References:
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010.
FAQs/MCQs:
What is claisen rearrangement?
Claisen rearrangement is the sigmatropic rearrangement of allyl vinyl ether or allyl aryl ethers to form γ,δ– unsaturated carbonyl compound or O-allyl substituted phenols respectively.






