Table of Contents
TogglePolythiazyl, also known as polymeric sulfur nitride is a gold or bronze-colored polymer with a metallic shine and is electrically conductive. In addition to being the first conductive inorganic polymer identified, it was also shown to be a superconductor at very low temperatures. It has the same physical properties as metals. Depending on the sample’s orientation, it is a fibrous solid that is described as lustrous golden on the faces and dark blue-black. it is insoluble in all solvents and air-stable.
Structure and Bonding
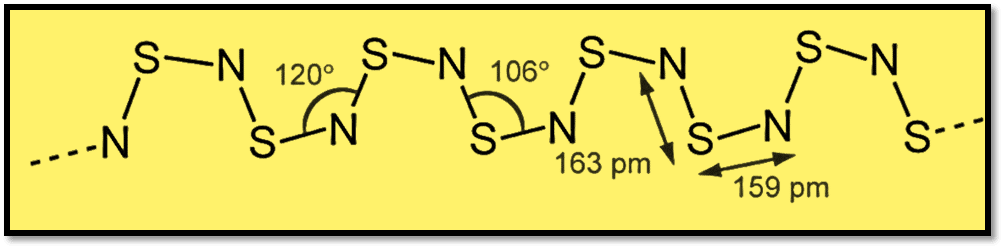
It is a polymer made of trivalent nitrogen, divalent sulfur, and tetravalent sulfur. The S and N atoms in neighboring chains line up. It is possible to write many resonance structures.
By using X-ray diffraction, the crystalline compound’s structure was clarified. This displayed S-N bond lengths of 159 pm and 163 pm in alteration, as well as 120-degree S-N-S bond angles and 106-degree N-S-N bond angles.
Polythiazyl preparation
Polythiazyl is synthesized by the polymerization of the dimer disulfur dinitride, which in turn is synthesized from the cyclic alternating tetramer tetrasulfur tetranitride. Conversion from the cyclic tetramer to dimer is catalyzed with hot silver wool. S2N2, then polymerizes slowly to form polythiazyl (SN)x. This is a bronze colored shiny solid that looks like a metal.
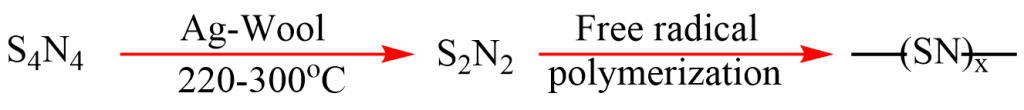
Polythiazyl structure
The structure of S4N4 is a heterocyclic ring. Electron diffraction and x-ray measurements have shown that this compound has an eight-membered cradle-shaped ring structure in which the average S-N bond length is 1.62 Å. Since the sum of the covalent radii for S and N is 1.7 Å, the S-N bonds seem to have some double bond character.
The fact that the bonds are of equal length suggests that this is delocalized. The S….S distance at the top and bottom of the cradle is 2.58 Å. The Vanderwall’s distance is 2.08 Å. This indicates weak S-S bonding and S4N4 is thus a cage structure.
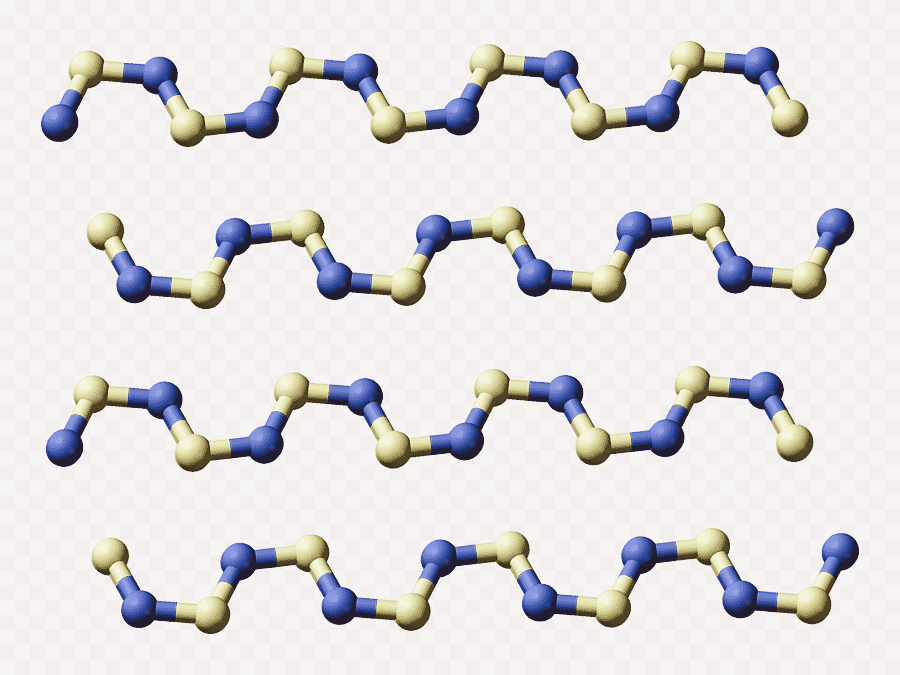
Polythiazyl as one dimensional conductor
The crystal structure shows that the four-membered rings in S2N2 have opened and polymerized into a long-chain polymer. A free radical mechanism has been suggested leading to a chain polymer. The atoms have a zig-zag arrangement and the chain is almost flat.
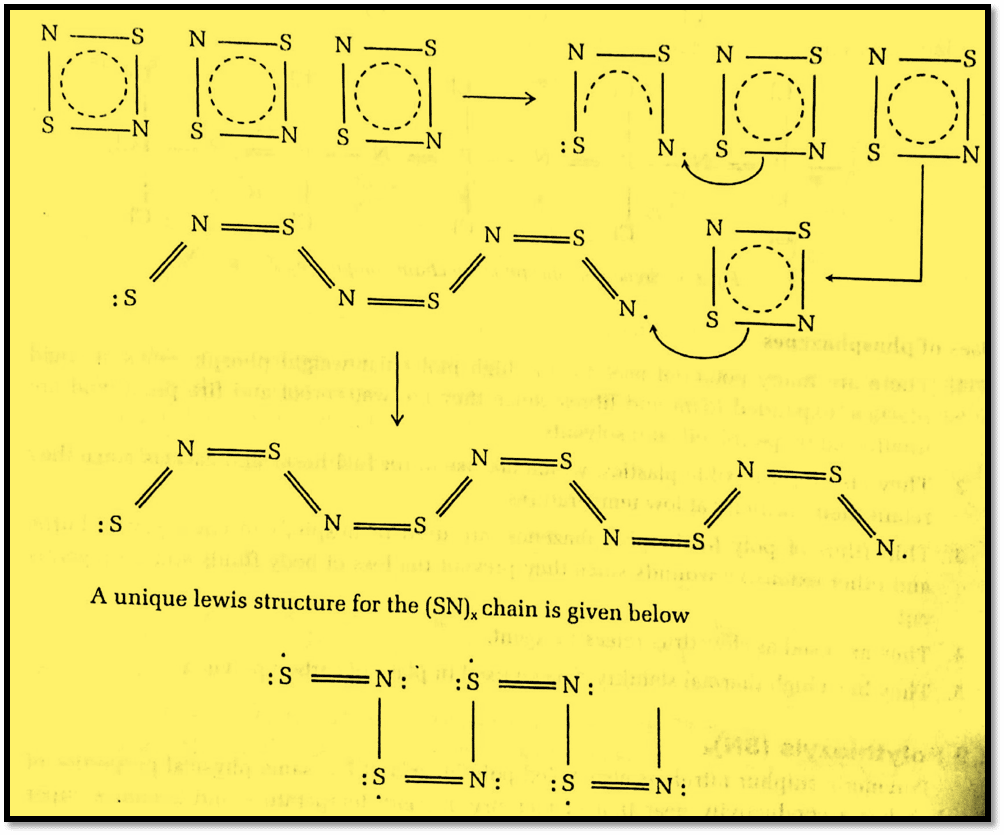
X-ray diffraction shows that in the SN chain, sulphur and nitrogen are in alternate positions. The Lewis structure illustrates many features of a conjugated single-bond and double-bond resonance system with nine electrons on each S atom rather than a lewis octet (8 electrons). Every S-N unit will have thus one antibonding electron. The half-filled overlapping antibonding orbitals will combine to form a half-filled conduction bond in much the same way as the half-filled 2s orbitals on a mole of Li atoms. But this conduction bond will lie only along the direction of (SN)x fiber and the polymer is thus termed a one-dimensional conductor or one-dimensional metal.
Uses of polythiazyls
When tetrasulfur tetranitride is vapourised under reduced pressure and passed through silver wool, disulfur dinitride is formed. The most important reaction of disulfur dinitride is the slow polymerization of the solid or vapor to form polythiazyls. It conducts electricity and conductivity increases as the temperature decreases, which is typical of metal. It becomes superconduction at 0.26K.
Due to this electrical conductivity, polythiazyls are used in LEDs, battery cathodes, transistors, and solar cells.
FAQS/MCQS
What are polythiazyls made of?
Polythiazyls are synthesized by the polymerization of the dimer disulfur dinitride, which in turn is synthesized from the cyclic alternating tetramer tetrasulfur tetranitride.
Is polythiazyl a one-dimensional conductor?
It acts as a one-dimensional metallic conductor because it has a 3D structure.
Which polymer of polythiazyls acts as a thermochromic compound?
S4N4 acts as a thermochromic compound.
What is the formula of tetrasulfur tetranitride?
The formula of tetrasulfur tetranitride is S4N4.
Why do we need inorganic polymers?
We need inorganic polymers in various fields such as in glasses, ceramics, rubber, and plastic. They are extensively used in petrochemical industries.






