Table of Contents
ToggleFrancium element is an alkali metal having an atomic number(Z) 87, belonging to the s-block element. Francium has the same number of valence electrons as lithium, thus these are kept in the same group IA.
Discovery of Francium element
Francium was discovered by French Scientist Marguerite Perey in 1939 in France. Before its discovery, it remained on the periodic table as only an element 87. The name francium was given to this element by M. Perey in honor of her native France.
Occurrence of francium element in nature
The element francium is the second-rarest naturally occurring element on Earth after Astatine(At). Due to its high radioactivity and instability, it is a difficult element to find and research in nature. However, francium must be seen as having a transitory existence naturally, even for a very extremely short period, because it is one of the daughters’ elements of Uranium (U235)
Fr223 is the most stable isotope of Francium among 34 other isotopes. This isotopes results from the alpha-decay of actinide isotope 227. 99% of the actinium atoms decay through beta-emission whereas 1% also undergo alpha decay.
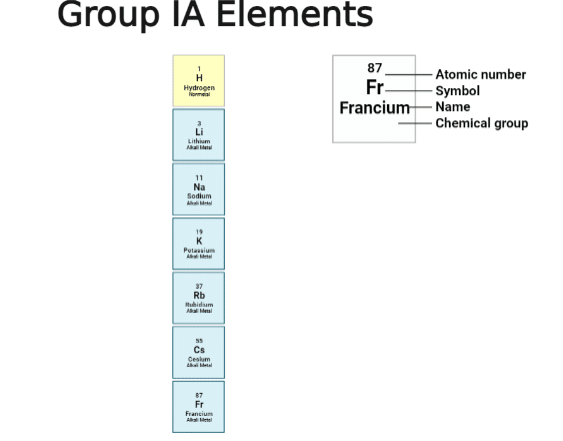
Francium electron configuration
The electronic configuration of francium(Fr87): 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s1
The noble gas configuration for francium: [Rn] 7s1, where Rn= Radon
Physical properties of francium
- Silvery metals with low melting points
- Soft and ductile
- Density is quite low
- Thermal and electrical conductivity is the highest of all known material
- Paramagnetic in nature due to a single unpaired S-electron.
- Compounds formed by Francium are characterized by relatively high melting and high boiling points.
| Atomic number | 87 | Group | 1 |
| Period | 7 | Melting point | 300 K (27 °C, 81 °F) |
| Boiling point | 950 K (677 °C, 1251 °F) | Density | Approx. 2.48 g/cm3 |
| Electronegativity | >0.79 (Pauling scale) | Ionization Energy | 393 kJ/mole (1st I.E) |
Chemical properties of francium
The electronic structure of the atoms provides a clear explanation for the chemistry and chemical characteristics of the elements as well as their compounds. For example; the metal’s low electron functions, low ionization potentials, and high position in the electromotive series all indicate the ease with which the valence electron is liberated.
why is francium the most reactive metal?
The electronic structure of francium has single weakly held valence s-electron and completely filled inner electron shells. It can easily lose valence electrons due to low electron work functions. Therefore, francium is the most reactive metal.
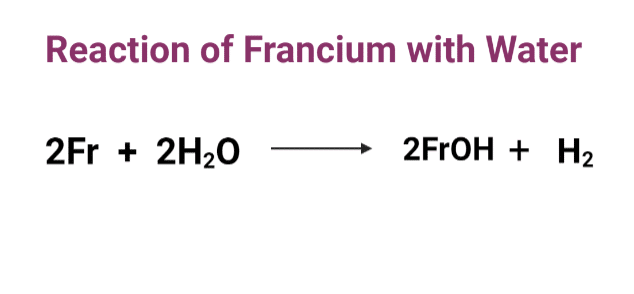
francium reaction with water
Francium reacts vigorously with water to liberate hydrogen gas and forms francium hydroxide. The reaction of francium is more violent with liquid water than with water vapor.
What is francium explosion?
If a small amount of francium is dropped in water, it reacts explosively producing huge sounds. This is known as a francium explosion.
francium bomb
Francium is a very reactive metal and it produces a bomb-like effect on reacting with water. Thus, francium can be used as a potential source of explosive materials such as bombs. This is called a francium bomb.
why francium is so expensive?
Francium is a very rare and unstable element on the Earth’s surface, making it one of the most costly elements to synthesize and obtain. The reasons for the expensiveness of the francium are listed below:
- It is the rarest element and it is produced by the radioactive decay of heavy elements such as Uranium.
- It has a very short half-life period and disintegrates into lighter elements, making its existence for a short time.
- Its production is very complex due to its radioactive nature.
Uses of Francium element
Francium has a very short half-life and hence it has no commercial applications known as of today. However, it can be used for scientific research. Moreover, it can be used in the diagnosis of cancer for its treatment.
Francium iodide
Francium iodide is a compound formed by francium and iodine. It has a molecular formula FrI with a molar mass of 349.9045. Due to the short existence of francium metal in nature, the probability of the formation of Francium iodide is very very low or may be impossible to find out this compound.
francium dropped in water youtube video
FAQs/MCQs
who discovered francium?
French Scientist Marguerite Perey discovered francium.
how many valence electrons does francium have?
Francium has only one valence electron.
where is francium found?
Due to its radioactive nature and very short half-life, francium is difficult to find on Earth’s crust.
when was francium discovered?
Francium was discovered in 1939 by French Scientist Marguerite Perey.
how many protons does francium have?
Francium has 87 protons.
how many neutrons does francium have?
Francium has 136 neutrons.
how many electrons does francium have?
A Francium atom has 87 electrons.
how many energy levels does francium have?
Francium has 7 energy levels. ( K, L, M, N, O, P, Q)
is francium a metal nonmetal or metalloid?
No, francium is an alkali metal.
is francium flammable?
No, francium is not flammable. Instead, it is very reactive.
is francium magnetic?
Francium is not magnetic in nature, but it is paramagnetic.
is francium the most reactive metal?
Yes, francium is the most reactive metal.
how is francium stored?
Francium can be stored in special apparatus made up of glass.






