Table of Contents
ToggleBoranes, or neutral boron hydrides, are cage compounds formed of boron and hydrogen. These are the compounds with the formula BxHy and related anions. For example, B2H6, B4H10, B5H9, B5H11, B6H10, and B10H14.
Structure of borane
In boranes, the B atom is bonded to at least one hydrogen atom. Some hydrogen atoms are bonded to two boron B atoms and are called bridging H. The hydrogen atom , which is bonded to a single B atom, is called terminal H. The structure of several boranes is illustrated below; there are typically three different structural types possible:
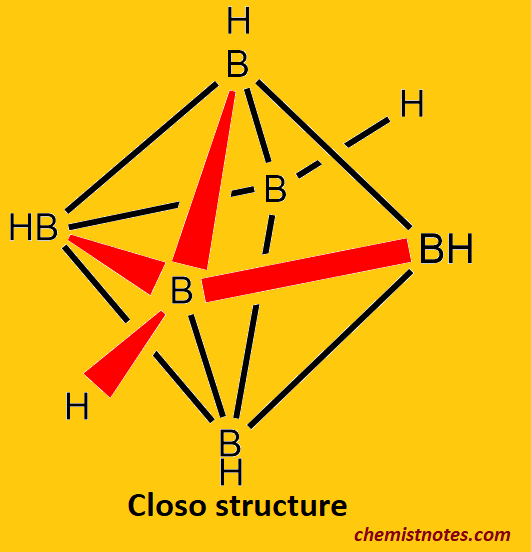
Closo-boranes
- One of the three main structures of borane, which has the chemical formula BnHn2-, is closo borane.
- Closo borane is thermally stable and moderately reactive.
- example: [B5H5]2−: the ion builds up a trigonal, bipyramidal polyhedron
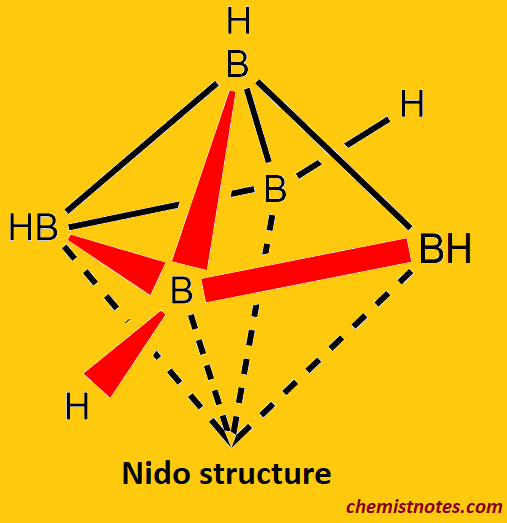
Nido-boranes
- Nido borane is a type of borane structure having the chemical formula BnHn+4.
- In nido borane thermal stability lies between closo- and arachno-boranes.
- example: B5H9 its structure can be assumed as the octahedral deltahedron of [B6H6]2− without one corner tetragonal pyramid.
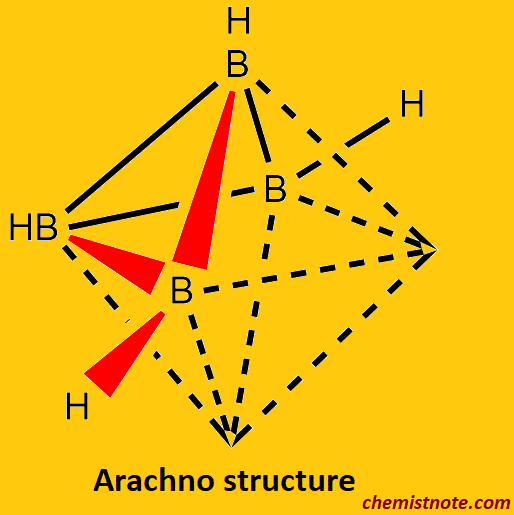
Arachno-boranes
- Arachno borane is a type of borane structure that has the chemical formula BnHn+6.
- Arachno borane is thermally unstable at room temperature and highly reactive.
- example: {B4H10}
There exist also other structures like the hypho-boranes, but they are less important.
Diborane (B2H6)
In a molecule of diborane (B2H6), there are not enough valence electrons to form the expected number of normal covalent bonds between all the atoms. Since the B atom has three electrons in the outermost shell, it can link with three hydrogen atoms. Thus, while each of the B atoms in diborane can link itself to three H-atoms, there are no electrons to form a bond between two B-atoms. Hence, it was clear that B2H6 contains 12 valance electrons and its valance electrons are insufficient for an ethane-like (H3B-BH3) structure that has seven bonds and would require 14 electrons. Hence, B2H6 is also called an electron-deficient compound.
Strucrure and Bonding in dibroane
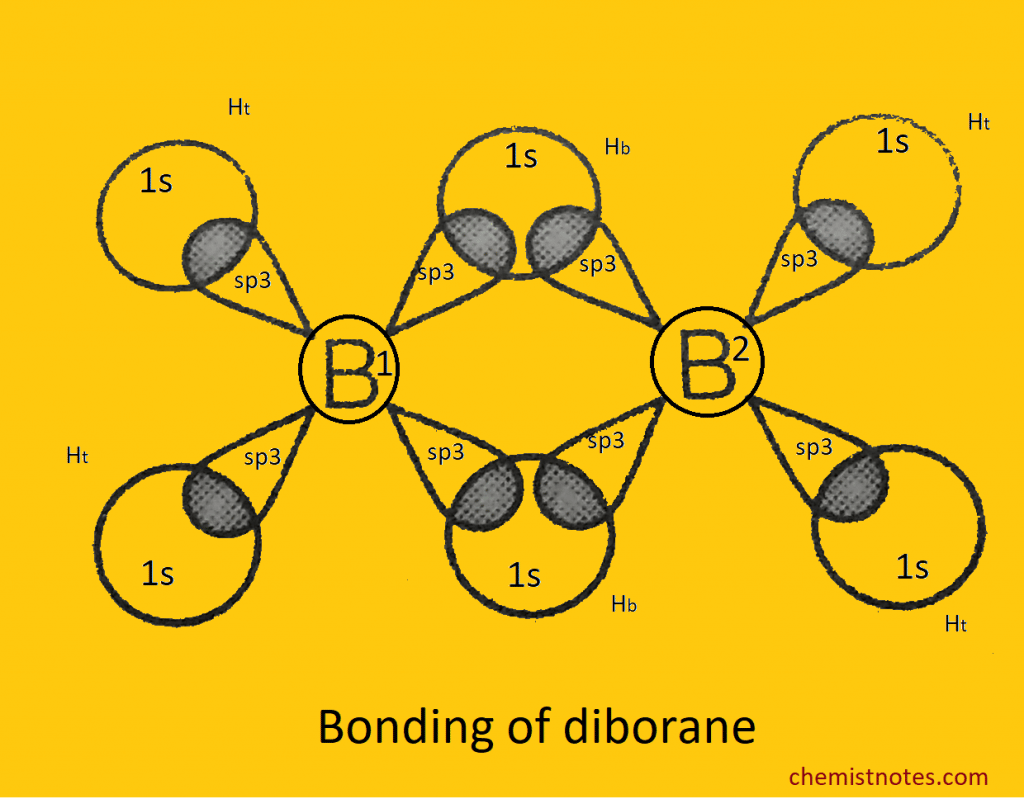
Electron diffraction and IR study shows that B2H6 has a hydrogen-bridge structure as like below:
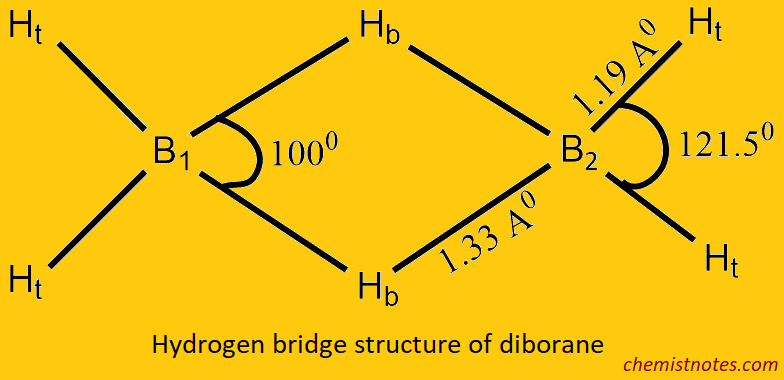
Its 12 valance electrons are insufficient to form the normal 2 electron 2 centered (2e–2c) bonds, so that each boron atom is bonded to two terminal hydrogen atoms (Ht) and the other two with a common hydrogen bridge (Hb) and forms the banana bond called the 2 electron 3 (2e–3c) centered bond. The valance configuration of the boron atom at G.S. 2s2, 2px1, 2p0y, 2p0z and its exited becomes 2s1, 2px1, 2py1, 2pz0. The four atomic orbitals hybridized together to give four sp3 hybrid orbitals in which one is empty, and the remaining three are singly filled. Hence, each boron atom uses sp3 hybrid orbitals.
One singly filled sp3 hybrid orbital on B1 atom, one empty sp3 hybrid orbital on B2 atom and one singly filled 1s atomic orbital on one Hb atom overlap together and form another bridging 2e–-3c bond, B1-Hb-B2 bond.
Similarly, one empty sp3 hydrid orbital B1 atom, one singly filled sp3 hybrid orbital on B2 atom and one singly filled 1s orbital on other Hb atom overlap together and form other bridging 2e–-3c bond.
Remaining two singly filled sp3 hybrid orbitals of each B-atom overlap with singly filled 1s orbital of Ht atom to form normal 2e–– 2c bonds.
FAQs
What are boranes?
Boranes, or neutral boron hydrides, are cage compounds formed of boron and hydrogen.






