Table of Contents
ToggleNorthern blotting is a scientific method for identifying and examining particular RNA molecules in a sample. Its resemblance to the Southern blotting method, used to find DNA, gave rise to its name. Just like other blotting techniques, it is used to detect particular RNA like southern blotting is for the detection of DNA, and where western blotting is for the detection of proteins.
Northern Blotting definition
When performing Northern blotting, RNA is obtained from a sample, size-separated on a gel, and then transferred to a membrane. A tagged probe complementary to the desired RNA sequence is used to detect the RNA afterward. A signal that may be seen using autoradiography or other imaging techniques develops when the labeled probe hybridizes with the RNA on the membrane.
RNA processing, gene expression, and the discovery of new RNA molecules can all be studied using northern blotting. Although newer, more sensitive methods like quantitative PCR and RNA sequencing have completely replaced it, it is still useful in some applications.
Northern Blotting principle
The fundamental idea behind Northern blotting is the separation of RNA into different sizes, which is then detected on a membrane using a hybridization probe with a base sequence complementary to all or a portion of the sequence of the target mRNA.
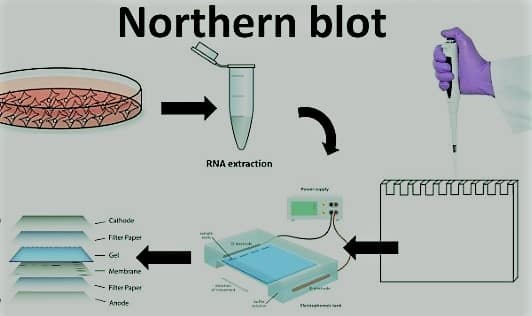
Northern Blotting Protocol
- RNA isolation: Using a standard procedure like TRIzol or a commercial RNA isolation kit, total RNA from the sample is extracted. Using spectrophotometry and gel electrophoresis to calculate the amount of RNA and its presence and evaluate its quality.
- RNA sample preparation: The RNA sample is prepared by heating it in a denaturing solution that contains formaldehyde and glyoxal. This will disrupt the RNA’s secondary structure and stop it from re-annealing. The RNA is then separated by size using an agarose gel that contains formaldehyde. After the gel has been stained with ethidium bromide, the RNA should be seen under UV lighting.
- Transfer of RNA into membrane: Capillary transfer, electroblotting, or vacuum-assisted transfer of the RNA from the gel to a positively charged nylon or nitrocellulose membrane are all acceptable methods. The RNA has been baked or UV cross-linked onto the membrane.
- Prehybridization: To reduce the probe’s nonspecific binding, block the membrane in a prehybridization solution containing blocking agents, such as salmon sperm DNA or non-fat dry milk.
- Probe hybridization: The tagged probe complementary to the target RNA to hybridize the membrane is used. A non-radioactive reporter, such as biotin or digoxigenin, is used to label the probe instead of being radiolabeled. In a hybridization buffer containing the probe and denaturants, hybridization is typically conducted overnight at a moderate temperature.
- Post-hybridization washing: To get rid of any unbound probe and the background signal is reduced, and then is washed the membrane with a series of washing buffers.
- Detection: By using chemiluminescence, colorimetry, or autoradiography the tagged probe on the membrane is find out. The signal is visualized with an imaging device that uses chemiluminescence, colorimetry, or X-ray film.
Northern Blotting Vs other Blotting Techniques
Northern blotting differs from other blotting methods in that it has both benefits and drawbacks. As it uses a tagged probe that is complementary to the RNA of interest, one benefit is that it enables the detection of particular RNA molecules. This makes it a great tool for researching RNA-related processes like RNA processing and gene expression.
The fact that it can be used to find both small and mRNA molecules is an additional benefit. Northern blotting does have certain drawbacks, though. One is that it needs considerable amounts of RNA, which in some circumstances might be challenging to obtain. Another drawback is that, in comparison to more recent techniques like qPCR and RNA sequencing, it requires a lot of time and labor.
Overall, Northern blotting is still a valuable method for examining RNA expression and processing, but other methods with higher sensitivity, throughput, and convenience have essentially replaced it.
Application of Northern Blotting
- Gene expression
- The expression of a certain gene can be examined using Northern blotting in several tissues or in laboratory conditions. This method can also be used to study alternative splicing, in which the same gene produces distinct isoforms depending on the tissue or stage of development.
- RNA quantification
- One method for determining the quantity of a particular RNA transcript in a sample is northern blotting. This may be helpful for examining how gene expression levels fluctuate over time or when comparing the gene expression of healthy and sick tissues.
- Detection of RNA editing
- When particular nucleotides in RNA molecules are altered post-transcriptionally, RNA editing events can be found using northern blotting. Understanding the functional effects of RNA editing and its function in gene regulation can benefit from this.